肝细胞核因子(hepatocyte nuclear factors, HNFs)是一组在肝细胞中优势表达的转录因子,其相互作用并构成强大的转录因子网络系统,在促进肝细胞分化和功能维持方面发挥重要作用[1-2]。HNF1α是HNFs家族的主要成员之一,在分化成熟的肝细胞中高表达,并在肝脏早期发育中起关键作用[3]。研究表明HNF1α可结合至少222个肝脏靶基因顺式作用元件,其转录调控的靶基因参与肝酶合成及代谢反应的不同阶段,维持肝细胞的正常功能[4]。
本课题组前期研究发现,HNF1α在肝纤维化患者和大鼠肝组织中表达均下降,在二甲基亚硝胺(DMN)或胆管结扎(BDL)构建的大鼠肝纤维化模型中,利用腺病毒AdHNF1α上调肝脏HNF1α的表达可缓解大鼠的肝纤维化进展[5]。由于腺病毒可介导肝脏中多种细胞过表达HNF1α,而内源性的HNF1α仅在肝细胞中表达,因此,HNF1α是否可通过特异性地改善肝细胞功能抑制肝纤维化仍有待进一步明确。甲状腺素结合球蛋白(thyroxine-binding globulin, TBG)为成熟肝细胞特异表达蛋白,已有研究表明,携带TBG启动子和内含子的腺相关病毒(AAV8-TBG)可驱动下游基因在成熟肝细胞中特异表达[6]。本研究在四氯化碳(CCl4)诱导的小鼠肝纤维化模型中,利用AAV8-TBG-HNF1α特异性上调肝细胞HNF1α表达,观察肝细胞中HNF1α的表达对小鼠肝纤维化的作用。
1 材料和方法 1.1 实验动物随机选取18只同系雄性清洁级C57/B6小鼠,8周龄,体质量16~18 g,委托第二军医大学实验动物中心培育[许可证号:SYXK(沪)2012-0003]。
1.2 病毒与试剂通过携带TBG启动子的腺相关病毒载体pENN-AAV-TBG-PI-RBG(Penn Vector Core, p1015-R)构建可特异性上调肝细胞HNF1α表达的腺相关病毒AAV8-TBG-HNF1α,病毒的包装及纯化均由深圳百恩维生物科技有限公司完成。CCl4购自国药集团化学试剂有限公司(纯度≥99.5%,进口分装),天狼猩红试剂购自Sigma公司。TRIzol试剂购自Invitrogen公司;反转录酶链反应试剂盒和SYBR® Green PCR试剂盒均购自TaKaRa公司,PCR引物由华大基因公司合成。HNF1α兔多克隆抗体购自Abcam公司,α-平滑肌肌动蛋白(α-SMA)小鼠单克隆抗体购自Boster公司,TUNEL试剂盒购自碧云天生物技术公司。
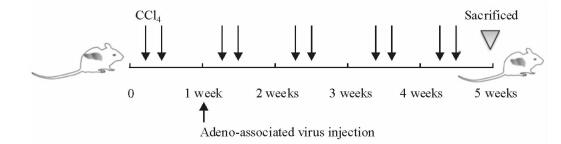
1.3 小鼠肝纤维化模型的构建及给药将18只小鼠随机分为正常组、对照病毒造模组(AAV8-TBG-Ctrl组)、治疗病毒造模组(AAV8-TBG-HNF1α组),每组6只。对照组及治疗组小鼠腹腔注射33.3% CCl4(按植物油:CCl4=2:1配制)建立小鼠肝纤维化模型,注射剂量为1 μL/g,每周注射2次,连续5周。在首次CCl4处理1周后,两组小鼠分别通过尾静脉注射腺相关病毒AAV8-TBG或AAV8-TBG-HNF1α,剂量为2×1011 Vg/只(Vg表示AAV病毒滴度),待造模结束后,麻醉处死小鼠(图 1)。取每只小鼠同部位完整肝叶组织,充分浸泡于10%中性甲醛中固定、石蜡包埋,用于后续天狼猩红染色、苏木精-伊红(H-E)染色、免疫组化及TUNEL实验;其余肝脏部分快速剪碎,存储于标记好的1.5 mL离心管中并置于液氮速冻,后转移至-80 ℃冰箱中长期保存,用于提取组织RNA。
|
图 1 实验性小鼠肝纤维化模型造模过程示意图 Fig 1 Schematic representation of induction of experimental hepatic fibrosis in mice |
1.4 免疫组织化学法检测肝组织中HNF1α、α-SMA和Ki-67的表达
取石蜡包埋的肝组织进行连续切片,厚度为4 μm,并附着于载破片上,通过链霉亲和素-过氧化物酶(streptavidin-peroxidase,SP)连结法进行免疫组化染色,在光学显微镜(Axiovert 40 CFL,ZEISS)下观察HNF1α、α-SMA和Ki-67表达。
1.5 肝脏的组织学检查取石蜡包埋的肝组织用切片机切为3 μm厚的组织片,并附着于载玻片上,分别使用H-E染色法和天狼猩红染色法对组织切片进行染色,在光学显微镜(Axiovert 40 CFL,ZEISS)下观察肝脏的组织形态和肝组织的胶原沉积情况。
1.6 qPCR法检测肝组织中纤维化相关基因、上皮指标及间质指标的变化将肝组织研成粉末,用TRIzol试剂提取各组小鼠肝组织中的RNA,使用紫外分光光度计(NANODROP2000,Thermo Scientific)检测RNA的纯度和浓度,应用反转录酶链反应试剂盒合成cDNA,反应条件:37 ℃, 15 min; 85 ℃, 30 s; 4 ℃,∞。cDNA产物于-20 ℃中保存。随后应用SYBR® Green PCR试剂盒检测HNF1α及肝纤维相关基因、上皮指标及间质指标的表达,PCR反应条件:94 ℃, 30 s; 94 ℃, 10 s; 60 ℃, 30 s; 连续扩增40个循环后检测熔解曲线。以β-actin为内参,比较目的基因和β-actin的Ct值。各基因的引物序列见表 1。
|
|
表 1 qPCR检测基因的引物序列 Tab 1 Primers for qPCR |
1.7 TUNEL法检测肝组织的细胞凋亡情况
配制20 μg/mL不含DNase的蛋白酶K,对肝组织切片进行抗原修复,37 ℃孵箱中作用20 min;滴加现配的TUNEL检测液,保持切片的湿润,37 ℃孵箱中避光孵育60 min,用含有DAPI的抗荧光淬灭封片液进行封片,共聚焦荧光显微镜(SP5,Leica)观察标本的TUNEL阳性细胞数,判断细胞的凋亡程度。
1.8 统计学处理采用SPSS 16.0软件进行数据分析,数据以x±s表示,两组间比较采用非配对t检验。检验水准(α)为0. 05。
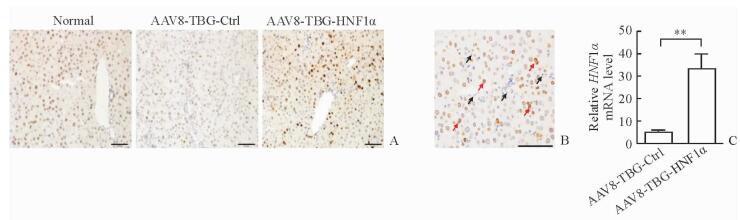
2 结果 2.1 病毒AAV8-TBG-HNF1α特异性上调肝细胞HNF1α表达免疫组化结果(图 2A)显示,与正常组小鼠相比,经CCl4造模后小鼠肝脏肝细胞中HNF1α的表达下调,而注射AAV8-TBG-HNF1α可上调小鼠肝脏肝细胞中HNF1α的表达。进一步放大免疫组化染色图片(图 2B)可见,AAV8-TBG-HNF1α组小鼠肝细胞胞核中HNF1α染色加深,而肝星形细胞(hepatic stellate cells,HSCs)、胆管细胞、内皮细胞和Kuppfer细胞等非实质细胞的细胞核染色未见明显变化。qPCR结果(图 2C)显示,AAV8-TBG-HNF1α组小鼠肝脏HNF1α mRNA的表达水平相比AAV8-TBG-Ctrl组升高(P<0.01),变化趋势与免疫组化结果相符。
|
图 2 AAV8-TBG-HNF1α特异性上调小鼠肝细胞HNF1α表达 Fig 2 Hepatocyte-specific up-regulation of HNF1α in mice mediated by AAV8-TBG-HNF1α A: Immunohistochemical staining of HNF1α in normal livers and fibrotic livers treated with AAV8-TBG/AAV8-TBG-HNF1α; B: Immunohistochemistry for fibrotic mice after AAV8-TBG-HNF1α injection, more HNF1α was located in hepatocytes (red arrow) but not in non-parenchymal cells (black arrow); C: Expression of HNF1α mRNA in livers analyzed by qPCR. AAV: Adeno-associated virus; TBG: Thyroid-binding globulin; Ctrl: Control; HNF1α: Hepatocyte nuclear factor 1α. Scale bars=100 μm (A, B). **P < 0.01. n=6, x±s |
2.2 特异性上调肝细胞HNF1α表达对小鼠肝纤维化的治疗作用
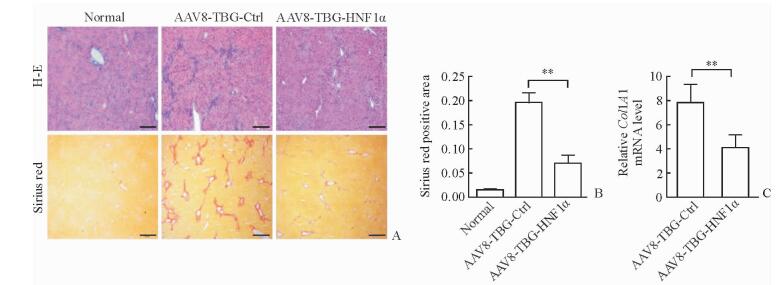
H-E及天狼猩红染色结果(图 3A)显示,与正常组小鼠相比,CCl4造模后小鼠肝组织结构紊乱,汇管区周围结缔组织增生明显,组织中可见大量炎性细胞浸润,且红色胶原纤维明显增粗变长。与AAV8-TBG-Ctrl组相比,AAV8-TBG-HNF1α组小鼠结缔组织条索减少,炎性细胞浸润减轻,同时肝脏中胶原沉积降低;胶原定量分析结果显示两组间差异有统计学意义(P<0.01,图 3B)。与AAV8-TBG-Ctrl组相比,AAV8-TBG-HNF1α组小鼠纤维化肝脏中COL1A1 mRNA的表达水平降低(P<0.01,图 3C),其变化与免疫组化结果一致。
|
图 3 特异性上调肝细胞HNF1α表达改善小鼠肝纤维化 Fig 3 Hepatocyte-specific up-regulation of HNF1α improves liver fibrosis in mice A: Liver morphology in mice in the normal, AAV8-TBG-Ctrl and AAV8-TBG-HNF1α groups was determined by H-E and sirius red staining; B: Semi-quantitative analysis of sirius red; C: Relative expression of COL1A1 mRNA in mice livers analyzed by qPCR. AAV: Adeno-associated virus; TBG: Thyroid-binding globulin; Ctrl: Control; HNF1α: Hepatocyte nuclear factor 1α; COL1A1: TypeⅠcollagen α1 chain. Scale bars=100 μm (A). **P < 0.01. n=6, x±s |
2.3 特异性上调肝细胞HNF1α表达对HSCs活化的影响
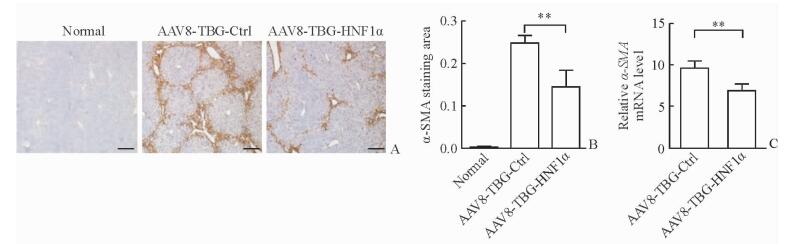
免疫组化结果(图 4A)显示,CCl4造模后小鼠肝组织中肝纤维化指标α-SMA的表达增高,而注射AAV8-TBG-HNF1α特异性上调肝细胞HNF1α的表达后,纤维化肝脏中的α-SMA的表达降低,与AAV8-TBG-Ctrl组相比差异有统计学意义(P<0.01,图 4B)。此外,与AAV8-TBG-Ctrl组相比,AAV8-TBG-HNF1α组小鼠肝脏中α-SMA mRNA的表达水平也下降(图 4C),说明特异性上调肝细胞HNF1α的表达可抑制HSCs的活化。
|
图 4 特异性上调肝细胞HNF1α表达抑制HSCs活化 Fig 4 Hepatocyte-specific up-regulation of HNF1α inhibits HSCs activation A: Expression of α-SMA in paraffin-embedded hepatic tissues by immunohistochemistry; B: Quantitation of α-SMA staining; C: Relative expression of α-SMA mRNA in mouse livers by qPCR. AAV: Adeno-associated virus; TBG: Thyroid-binding globulin; Ctrl: Control; HNF1α: Hepatocyte nuclear factor 1α; HSCs: Hepatic stellate cells; α-SMA: α-Smooth muscle actin. Scale bars=100 μm (A). **P < 0.01. n=6, x±s |
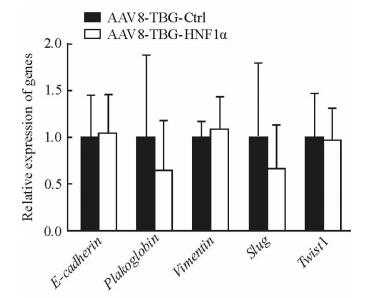
2.4 特异性上调肝细胞HNF1α表达对纤维化肝脏中上皮间质转换(epithelial-mesenchymal transition, EMT)进程的影响
用qPCR检测上皮指标(E-cadherin和Plakoglobin)及间质指标(Vimentin、Slug和Twist1) 等EMT指标的表达变化,结果(图 5)显示,AAV8-TBG-HNF1α组小鼠纤维化肝脏中EMT相关指标的表达与AAV8-TBG-Ctrl组相比差异均无统计学意义(P>0.05),表明HNF1α在调控肝纤维化进程时并不影响纤维化肝脏的EMT进程。
|
图 5 特异性上调HNF1α对小鼠纤维化肝脏EMT进程的影响 Fig 5 Effect of hepatocyte-specific up-regulation of HNF1α on EMT in fibrotic livers in mice AAV: Adeno-associated virus; TBG: Thyroid-binding globulin; Ctrl: Control; HNF1α: Hepatocyte nuclear factor 1α; EMT: Epithelial-mesenchymal transition. n=6, x±s |
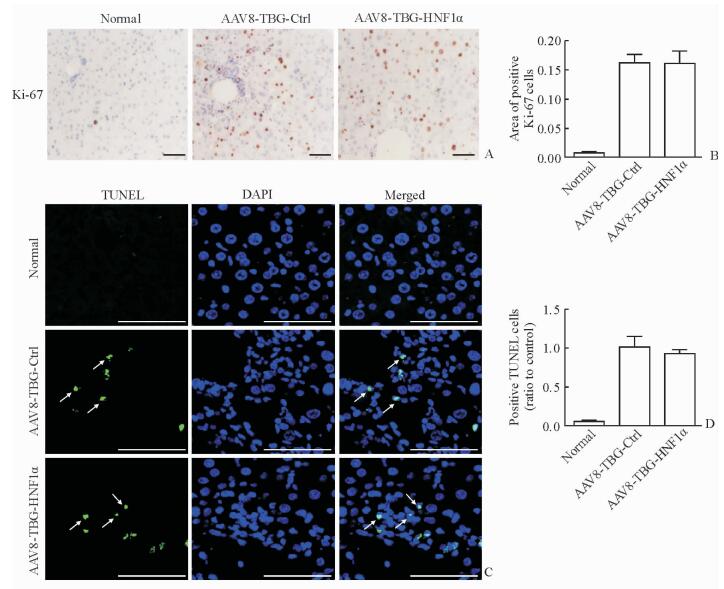
2.5 特异性上调肝细胞HNF1α表达对肝脏细胞增殖、凋亡的影响
AAV8-TBG-HNF1α组小鼠纤维化肝脏中的Ki-67阳性细胞数(图 6A、6B)和细胞凋亡数(图 6C、6D)与AAV8-TBG-Ctrl组相比差异均无统计学意义(P>0.05),表明特异性上调肝细胞中HNF1α的表达可能并非通过促进肝细胞增殖、减少肝细胞凋亡改善肝纤维化。
|
图 6 特异性上调HNF1α对肝细胞增殖与凋亡的影响 Fig 6 Effect of hepatocyte-specific up-regulation of HNF1α on liver cell proliferation and apoptosis A: Expression of Ki-67 in paraffin-embedded liver sections analyzed by immunohistochemistry; B: Positive Ki-67 cells analyzed by semi-quantitative analysis; C: Liver tissues were co-stained with DAPI (blue) and TUNEL (green, arrows) and visualized under confocal microscopy; D: TUNEL-positive cells analyzed by semi-quantitative analysis. AAV: Adeno-associated virus; TBG: Thyroid-binding globulin; Ctrl: Control; HNF1α: Hepatocyte nuclear factors 1α; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick-end labeling; DAPI: 4’, 6-Diamidino-2-phenylindole. Scale bars=100 μm (A, C). n=6, x±s |
3 讨论
肝纤维化是肝脏针对不同慢性损伤的修复反应,其进一步发展可引起假小叶形成,导致肝硬化,部分患者可发展成肝癌[7-8]。肝纤维化属于可逆性病变,尽早采取干预及治疗措施有望逆转,可为临床慢性肝病患者带来福音。既往研究认为,HSCs的激活是肝纤维化发生的中心环节,肝损伤时产生的细胞外基质(extracellular matrix, ECM)主要来源于活化的HSCs,而HSCs激活可能是细胞与细胞、ECM与细胞相互作用的结果[9-10]。近年来研究发现,肝细胞作为肝脏最主要的细胞,其细胞功能的完整性对维持肝脏稳态及再生至关重要[11],已逐渐成为急慢性肝病的研究热点。
既往研究发现,HNF4α可通过保护肝纤维化过程中的肝细胞功能和抑制活化的HSCs缓解肝纤维化进程,故提出了HNFs治疗肝纤维化的新策略[12]。HNF1α是HNFs家族中的重要成员,在肝脏发育、肝细胞分化及维持肝细胞生物学功能方面发挥重要作用[3-4, 13]。本课题组前期研究发现,在DMN或BDL诱导的大鼠肝纤维化模型中,肝细胞和HSCs之间存在炎症因子介导的交互作用,下调肝脏HNF1α可促进肝纤维化的发生和发展,而利用腺病毒AdHNF1α上调肝脏HNF1α的表达可抑制肝细胞炎症通路活化,维持肝细胞功能,并影响HSCs活化,进而改善肝纤维化,该结果证明了肝脏中HNF1α的抗纤维化效应,并提出维持肝细胞功能对改善大鼠肝纤维化的重要意义[5]。近期,本课题组构建了HNF1α肝细胞特异性基因敲除模式小鼠,发现肝细胞HNF1α基因缺失可诱发肝细胞自发脂肪变,并引起肝纤维化,最终导致肝癌发生,进一步说明肝细胞HNF1α表达与肝纤维化间的关系密切[4-6]。鉴于内源性HNF1α仅在肝细胞中表达,为明确HNF1α是否可通过特异性改善肝细胞功能治疗肝纤维化,本研究以CCl4诱导的小鼠肝纤维化模型为研究对象,探讨特异性上调肝细胞HNF1α的表达对肝纤维化的治疗作用。
近年来,AAV载体因其安全性好、可在体内长时间表达及低免疫原性等特点,被越来越多地运用于基因治疗的基础研究及动物实验中[14]。不同血清型的AAV对组织或细胞的嗜性不同,其中AAV8病毒具高嗜肝性,在肝脏疾病的基因治疗领域具有广泛的应用前景[15]。TBG为成熟肝细胞特异表达蛋白,有大量研究利用携带TBG启动子及内含子的腺相关病毒(AAV8-TBG)驱动下游基因在成熟肝细胞中特异表达[6]。Yanger等[16]利用腺相关病毒AAV8-TBG-Cre制备肝细胞谱系追踪小鼠,明确了成熟肝细胞在肝脏再生中作用。本研究应用AAV8-TBG-HNF1α特异性上调小鼠肝细胞中HNF1α的表达,在小鼠肝纤维化模型中证实了特异性上调肝细胞HNF1α表达可显著抑制纤维化肝脏中的结缔组织增生及胶原沉积,降低肝纤维化程度,提示AAV8-TBG-HNF1α用于肝纤维化治疗的可能性。本研究首次利用AAV介导HNFs在肝细胞中特异表达,并发现HNF1α对肝纤维化具有治疗作用,为未来HNFs治疗肝纤维化提供了新的手段。
在EMT进程中,上皮细胞逐渐失去上皮形态特征继而获得间质细胞特性。大量研究表明,EMT参与肝纤维化的形成,是肝纤维化发生过程中重要的致病机制[17]。有文献报道,在转化生长因子β(TGF-β)诱导的肝细胞EMT进程中伴随着HNFs表达水平的降低,包括HNF4α及HNF1α[18]。本课题组前期研究已证实HNF4α可抑制肝细胞及HSCs的EMT,从而缓解实验大鼠肝纤维化[12]。而本实验结果显示,特异性上调肝细胞HNF1α表达对纤维化肝脏的EMT进程未见明显影响,说明肝细胞HNF1α可能并非通过EMT影响肝纤维进程。
肝细胞为肝脏中最主要的组成细胞,在正常情况下很少分裂,但在肝脏过度损伤时可触发肝细胞增殖,以修复肝损伤[19]。本研究结果显示,特异性上调肝细胞HNF1α表达对纤维化肝脏中的肝细胞增殖无明显影响。肝细胞增殖和肝细胞凋亡间相互对立,肝细胞凋亡是肝损伤以及肝纤维化发生、发展过程中的一个重要特征,是目前肝病的研究热点[20]。特异性敲除肝细胞中Bcl-xL、Mcl-1或TAK1基因可诱发肝细胞凋亡,加重肝损伤,并通过细胞凋亡小体激活HSCs,进而促进肝纤维化[21-23]。本研究结果显示,特异性上调肝细胞HNF1α表达对HSCs的活化具有明显的抑制作用,而TUNEL染色结果显示在CCl4诱导的纤维化肝脏中仅有少量细胞凋亡,而HNF1α治疗组肝脏中的细胞凋亡未见明显改变,表明HNF1α对HSCs的抑制作用并非通过减轻肝细胞凋亡实现。由此,本实验结果进一步证实了改善肝细胞功能对HSCs活化的影响。
综上,特异性上调肝细胞HNF1α的表达可改善小鼠肝纤维化,为HNFs治疗肝纤维化提供了强有力的证据,并为未来临床抗肝纤维化治疗提供了新的思路。其更深入的分子生物学机制仍有待于今后进一步研究。
| [1] | NISHIKAWA T, BELL A, BROOKS J M, SETOYAMA K, MELIS M, HAN B, et al. Resetting the transcription factor network reverses terminal chronic hepatic failure[J]. J Clin Invest, 2015, 125: 1533–1544. DOI: 10.1172/JCI73137 |
| [2] | SCHREM H, KLEMPNAUER J, BORLAK J. Liver-enriched transcription factors in liver function and development. PartⅠ:the hepatocyte nuclear factor network and liver-specific gene expression[J]. Pharmacol Rev, 2002, 54: 129–158. DOI: 10.1124/pr.54.1.129 |
| [3] | PONTOGLIO M, BARRA J, HADCHOUEL M, DOYEN A, KRESS C, BACH J P, et al. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome[J]. Cell, 1996, 84: 575–585. DOI: 10.1016/S0092-8674(00)81033-8 |
| [4] | ODOM D T, ZIZLSPERGER N, GORDON D B, BELL G W, RINALDI N J, MURRAY H L, et al. Control of pancreas and liver gene expression by HNF transcription factors[J]. Science, 2004, 303: 1378–1381. DOI: 10.1126/science.1089769 |
| [5] | QIAN H, DENG X, HUANG Z W, WEI J, DING C H, FENG R X, et al. An HNF1α-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells[J]. Cell Res, 2015, 25: 930–945. DOI: 10.1038/cr.2015.84 |
| [6] | BELL P, GAO G, HASKINS M E, WANG L, SLEEPER M, WANG H, et al. Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs[J]. Hum Gene Ther, 2011, 22: 985–997. DOI: 10.1089/hum.2010.194 |
| [7] | LEE U E, FRIEDMAN S L. Mechanisms of hepatic fibrogenesis[J]. Best Pract Res Clin Gastroenterol, 2011, 25: 195–206. DOI: 10.1016/j.bpg.2011.02.005 |
| [8] | BATALLER R, BRENNER D A. Liver fibrosis[J]. J Clin Invest, 2005, 115: 209–218. DOI: 10.1172/JCI24282 |
| [9] | HELLERBRAND C. Hepatic stellate cells——the pericytes in the liver[J]. Pflugers Arch, 2013, 465: 775–778. DOI: 10.1007/s00424-012-1209-5 |
| [10] | BANSAL M B. Hepatic stellate cells:fibrogenic, regenerative or both Heterogeneity and context are key[J]. Hepatol Int, 2016, 10: 902–908. DOI: 10.1007/s12072-016-9758-x |
| [11] | MALATO Y, NAQVI S, SCHVRMANN N, NG R, WANG B, ZAPE J, et al. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration[J]. J Clin Invest, 2011, 121: 4850–4860. DOI: 10.1172/JCI59261 |
| [12] | YUE H Y, YIN C, HOU J L, ZENG X, CHEN Y X, ZHONG W, et al. Hepatocyte nuclear factor 4alpha attenuates hepatic fibrosis in rats[J]. Gut, 2010, 59: 236–246. DOI: 10.1136/gut.2008.174904 |
| [13] | NI Q, DING K, WANG K Q, HE J, YIN C, SHI J, et al. Deletion of HNF1α in hepatocytes results in fatty liver-related hepatocellular carcinoma in mice[J]. FEBS Lett, 2017, 591: 1947–1957. DOI: 10.1002/feb2.2017.591.issue-13 |
| [14] | MAK K Y, RAJAPAKSHA I G, ANGUS P W, HERATH C B. The Adeno-associated virus-a safe and effective vehicle for liver-specific gene therapy of inherited and non-inherited diseases[J]. Curr Gene Ther, 2017, 17: 4–16. |
| [15] | SEN D, GADKARI R A, SUDHA G, GABRIEL N, KUMAR Y S, SELOT R, et al. Targeted modifications in adeno-associated virus serotype 8 capsid improves its hepatic gene transfer efficiency in vivo[J]. Hum Gene Ther Methods, 2013, 24: 104–116. DOI: 10.1089/hgtb.2012.195 |
| [16] | YANGER K, KNIGIN D, ZONG Y, MAGGS L, GU G, AKIYAMA H, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation[J]. Cell Stem Cell, 2014, 15: 340–349. DOI: 10.1016/j.stem.2014.06.003 |
| [17] | IKEGAMI T, ZHANG Y, MATSUZAKI Y. Liver fibrosis:possible involvement of EMT[J]. Cells Tissues Organs, 2007, 185: 213–221. DOI: 10.1159/000101322 |
| [18] | TU X, ZHANG Y, ZHENG X, DENG J, LI H, KANG Z, et al. TGF-β-induced hepatocyte lincRNA-p21 contributes to liver fibrosis in mice[J]. Sci Rep, 2017, 7: 2957. DOI: 10.1038/s41598-017-03175-0 |
| [19] | FAUSTO N. Liver regeneration[J]. J Hepatol, 2000, 32(1 Suppl): 19–31. |
| [20] | HIGUCHI H, GORES G J. Mechanisms of liver injury:an overview[J]. Curr Mol Med, 2003, 3: 483–490. DOI: 10.2174/1566524033479528 |
| [21] | TAKEHARA T, TATSUMI T, SUZUKI T, RUCKER E B 3rd, HENNIGHAUSEN L, JINUSHI M, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses[J]. Gastroenterology, 2004, 127: 1189–1197. DOI: 10.1053/j.gastro.2004.07.019 |
| [22] | VICK B, WEBER A, URBANIK T, MAASS T, TEUFEL A, KRAMMER P H, et al. Knockout of myeloid cell leukemia-1 induces liver damage and increases apoptosis susceptibility of murine hepatocytes[J]. Hepatology, 2009, 49: 627–636. DOI: 10.1002/hep.22664 |
| [23] | INOKUCHI S, AOYAMA T, MIURA K, OSTERREICHER C H, KODAMA Y, MIYAI K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis[J]. Proc Natl Acad Sci USA, 2010, 107: 844–849. DOI: 10.1073/pnas.0909781107 |
 2017, Vol. 38
2017, Vol. 38


