颈椎后纵韧带骨化症(ossification of the posterior longitudinal ligament, OPLL)是由于颈椎后纵韧带异位成骨,骨化物压迫椎管内神经组织导致的疾病。该病在东亚人群中的发病率高于其他人群[1]。患者症状主要包括四肢与躯干感觉和运动功能障碍,严重者甚至出现瘫痪和大小便失禁[2-3]。目前对于OPLL的手术治疗策略尚无共识,通常认为,椎管狭窄率大于60%的严重OPLL采用后路减压手术治疗效果不佳[4-5]。而前路减压手术通过从前方切除骨化物实现神经减压,其减压效果直接,但切除骨化物时操作难度较高,面临的脑脊液漏和神经损伤的风险较大[6]。
我科针对严重OPLL设计了一种颈椎前路椎体骨化物复合体前移融合术(anterior controllable anteriodisplacement and fusion,ACAF),具有前路直接减压的手术疗效,同时具有后路减压的安全性。本文对该术式做一介绍,并对2例患者的初步疗效进行报道。
1 资料和方法 1.1 手术技术 1.1.1 术前检查术前进行颈椎正侧位平片、颈椎CT三维重建及颈椎磁共振成像等必要的影像学检查,通过颈椎平片判断患者颈椎曲度、颈部的长度和前方显露的可行性,颈椎磁共振成像判断脊髓受压的节段、所需减压的范围和脊髓所处的位置,颈椎CT三维重建测量骨化物的范围、宽度及厚度,增强CT可辅助判断椎动脉的走行和变异情况。
1.1.2 麻醉及体位采用全身麻醉,涉及C3椎体以上节段采用经鼻气管插管,C3椎体以下可采用经口气管插管。患者取仰卧位,肩下垫枕,并垫软垫使颈部略仰伸,不要过分后仰,以免导致气管张力过高而影响术中显露。常规进行神经电生理检测以保证术中操作的安全性。
1.1.3 显露采用Smith-Peterson入路显露椎前间隙(图 1A),根据手术节段长度及患者颈部条件决定选择横切口或纵切口。显露椎前间隙后,使用椎体钉对节段进行定位。使用双极电凝对前纵韧带上的血管进行预凝止血,用电刀及锐利的神经剥离子由骨膜下向外侧剥离颈长肌至钩椎关节外缘,再用电刀及骨蜡充分止血。

|
图 1 颈椎前路椎体骨化物复合体前移融合术操作步骤示意图 Fig 1 Schematic diagram of surgical procedures of anterior controllable anteriodisplacement and fusion surgery A: Huge ossification of posterior longitudinal ligament of cervical spine at the level C4-6; B: Discectomy of intervertebral space; C: Removal of the anterior part of centrum; D: Installation of titanium plate and screw; E, F: Bilateral osteotomies of the vertebrae; G, H: Antedisplacement of the vertebrae ossification complex |
1.1.4 处理椎间隙
使用三关节咬骨钳去除目标间隙及椎体前缘骨赘,依次使用尖刀、刮匙和髓核钳彻底去除椎间盘,用枪钳咬除椎间隙后缘增生骨赘,显露后纵韧带(图 1B)。使用神经剥离钩寻找并突破后纵韧带的薄弱点,然后挑起后纵韧带并用尖刀切开。使用刮匙及枪钳咬除椎间隙后纵韧带,显露硬脊膜。此时需注意,仅需切除头尾两端椎间隙的后纵韧带,骨化物所在的各节段椎间隙后纵韧带无需切除。
1.1.5 去除椎体前部骨质使用三关节咬骨钳根据各节段骨化物厚度去除椎体前部骨质,自体碎骨留作后续植骨用。预装一块钛板并使用测深器测量各椎体前缘与钛板间的距离,判断是否满足后续提拉所需空间。取下钛板,对前部骨质去除不足的椎体使用磨钻或超声骨刀进行修整(图 1C)。
1.1.6 安装钛板和椎间融合器根据试模测量各椎间隙大小,于各间隙安装填塞有自体骨的椎间融合器(注意融合器高度是否合适,不可强行敲入间隙,只可轻柔放入)。将预弯的合适长度的钛板放置于椎体前缘,用钻头及丝攻预处理钉道后,安装椎体钉(图 1D)。此处注意将需提拉的椎体上的椎体钉拧至贴合钛板即可,不做提拉尝试。
1.1.7 椎体两侧开槽根据术前测量骨化物的宽度,向外1 mm作为开槽边界,一般开槽宽度在18~20 mm(图 1E、1F),可使用高速磨钻、超声骨刀或咬骨钳进行开槽,槽宽1.5~2 mm,至椎体后壁皮质后,使用1 mm枪钳从两侧椎间隙向椎体中部逐渐咬除剩余椎体后壁。使用骨蜡及明胶海绵止血。
1.1.8 椎体骨化物复合体前移前述步骤完成后,椎体骨化物复合体与脊柱间的硬性连接即已断开。使用多把椎体钉起子同时拧紧需提拉节段的椎体钉,可观察到椎体逐渐前移。如未观察到椎体前移,需停止提拉,探查椎体骨化物复合体四周是否残留未断开骨质(图 1G、1H)。
1.1.9 关闭切口及术后制动仔细止血后冲洗术野,留置1~2根引流管,逐层关闭手术创口。术后使用头颈胸支具固定3个月,以保证术后早期活动及骨质融合。
1.2 病例介绍 1.2.1 病例1女性,57岁。主诉:颈部不适10余年,加重伴行走不稳1个月。患者于10年前无明显诱因出现颈部酸痛不适,无头痛、头晕,无恶心、呕吐,症状间断出现。自行局部理疗及针灸治疗,有所好转。1个多月前上述症状加重,伴双手麻木,行走不稳伴踩棉花感。专科检查:步入病室,步态不稳,脊柱呈生理弯曲,颈椎活动受限,双手手指末端触觉、痛温觉减退,位置觉、震动觉未见异常。四肢肌张力正常,肌肉无明显萎缩,双手有精细动作障碍,双上肢三角肌、肱二头肌、肱三头肌肌力4级,屈腕、伸腕肌力4-级,双手肌力4-级,双下肢髂腰肌、股四头肌、股二头肌、半腱肌、半膜肌、胫前肌、小腿三头肌肌力4级,双足趾背伸、跖屈肌力4-级。双侧腹壁反射正常,肛门反射正常,双侧肱二头肌、肱三头肌、桡骨膜反射亢进。双侧膝跳反射、跟腱反射亢进。双侧Hoffmann征阳性,双侧Babinski征阴性。术前颈椎侧位片示C2~7椎体后壁混合型后纵韧带骨化;颈椎CT三维重建示C2~7混合型OPLL,椎管狭窄率85%;颈椎磁共振成像示颈脊髓受压节段为C3~5、C6/7。诊断:(1) 颈椎OPLL伴不全瘫;(2) 颈椎管狭窄症;(3) 胸椎管黄韧带骨化。
1.2.2 病例2男性,69岁。主诉:双下肢无力伴双手麻木4年,大小便障碍2年,加重1个月。患者于4年前无明显诱因出现双手麻木,双下肢冰凉感伴双下肢无力、踩棉花感,行走不稳,伴摔倒倾向。未予特殊诊治。3年前不慎自电动车上摔下后症状明显加重,行走不稳,需搀扶行走,当地医院诊断为颈椎OPLL,建议手术治疗,患者拒绝。2年前出现胸部束带感及呼吸费力,下肢肌力进一步减弱,需助行器辅助行走,行走距离短;大便无力,小便需等待。1个月前双下肢无力症状明显加重,站立及行走困难。专科检查:轮椅入病室,锁骨及以下平面感觉障碍,四肢肌张力增强,以双下肢较为明显,体格检查时双下肢配合不佳。肌肉无明显萎缩,双上肢有精细动作障碍。双上肢三角肌、肱二头肌、肱三头肌肌力5级,屈腕、伸腕肌力5级,右手肌力4级,双下肢髂腰肌、股四头肌、股二头肌、半腱肌、半膜肌、胫前肌、小腿三头肌肌力3级,双足趾背伸、跖屈肌力3级。双侧腹壁反射未引出,双侧提睾反射正常,肛门反射减弱,双侧肱二头肌、肱三头肌、桡骨膜反射亢进。双侧膝跳反射、跟腱反射亢进。双侧Hoffmann征阳性,双侧Babinski征阳性。术前颈椎侧位片示颈椎曲度变直,各椎体不同程度骨质增生;CT矢状位示C3/4椎间隙后缘骨赘形成、C6~T1椎体后纵韧带连续性骨化、颈椎管狭窄率70%,横断面示C6~T1椎体后缘骨化物呈中央型向后突出、颈椎管严重狭窄;颈椎磁共振成像示C6~T1后纵韧带骨化导致椎管狭窄脊髓严重压迫,C3/4、C4/5、C5/6致压物呈中央型向后突出,压迫脊髓。诊断:(1) 颈胸椎OPLL(C6~T1)伴不全瘫; (2) 脊髓型颈椎病(C3/4、C4/5、C5/6)。
2 结果病例1行ACAF(C3~6前移,C2/3间骨化后纵韧带横断),手术时间5.5 h,术中出血800 mL。手术顺利,未发生脑脊液漏、神经功能恶化、术后血肿等并发症。术后影像学检查示C3~6椎体前移,椎管狭窄率由术前的85%降低为20%,脊髓压迫解除(图 2~4)。下肢肌力增加,行走功能显著恢复。术后JOA评分16分,术前JOA评分15分,JOA评分改善率为50.0%。
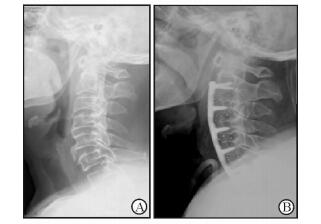
|
图 2 病例1术前及术后颈椎侧位片对比 Fig 2 Comparison of pre-and post-operative lateral fluoroscopy of case 1 A: Pre-operative lateral X-ray image showing a massive mixed type OPLL at C2-7; B: Post-operative lateral fluoroscopy showing the ossification was transected behind the C2/3 space, and the C3-6 ossification was hoisted and fused to an antedisplaced location. OPLL: Ossification of the posterior longitudinal ligament |
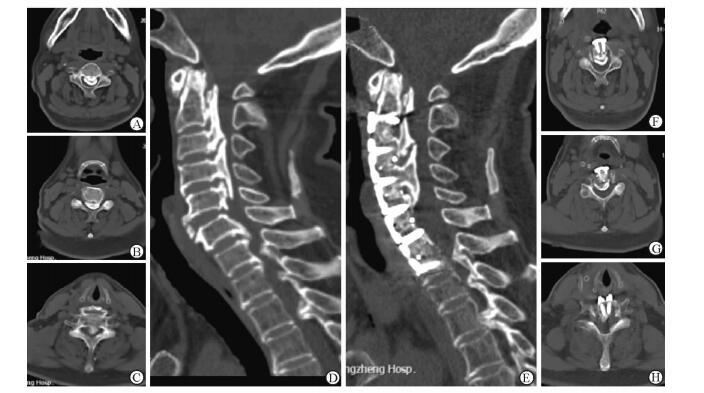
|
图 3 病例1术前及术后颈椎CT三维重建对比 Fig 3 Comparison of pre-and post-operative CT 3D reconstruction of case 1 A, F: C3 level of axial plane; B, G: C4 level of axial plane; C, H: C6 inferior endplate level of axial plane; D, E: Central sagittal plane. A-D: Pre-operative sagittal CT image showing a massive mixed type OPLL at C2-7, canal narrowing rate was 85%; E-H: Post-operative sagittal CT image showing the ossification was hoisted and fused to an antedisplaced location, the spine canal narrowing rate was 20%. OPLL: Ossification of the posterior longitudinal ligament |
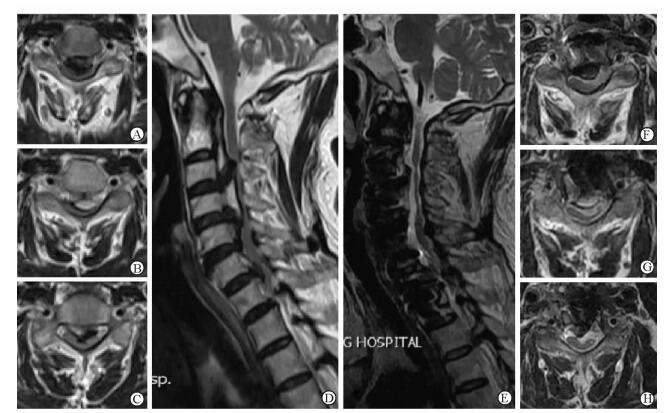
|
图 4 病例1术前及术后颈椎磁共振成像对比 Fig 4 Comparison of pre-and post-operative magnetic resonance imaging of case 1 A, F: C3 level of axial plane; B, G: C4 level of axial plane; C, H: C6 level of axial plane; D, E: Central sagittal plane. A-D: Pre-operative magnetic resonance imaging (MRI) showing severe compression of spinal aord at the level C3-5 and C6/7 space; E-H: Post-operative MRI showing the decompression of cervical spinal cord in each level; the morphology of spinal cord did not fully recover |
病例2行前路颈椎间盘切除椎间植骨融合(C3/4)加ACAF(C5~7椎体前移,T1上半椎体后缘潜行减压),手术时间4.8 h,术中出血500 mL。手术顺利,未发生脑脊液漏、神经功能恶化、术后血肿等并发症。术后影像学检查示C5~7椎体前移,椎管狭窄率由术前的80%降低为5%,脊髓压迫解除(图 5~7)。术后躯干部束带感消失,双上肢、下肢肌力增加,行走功能显著恢复,术后JOA评分14分,术前JOA评分6分,JOA评分改善率为72.7%。

|
图 5 病例2术前及术后颈椎侧位片对比 Fig 5 Comparison of pre-and post-operative lateral fluoroscopy of case 2 A: Pre-operative lateral fluoroscopy showing degeneration and a decrease in the lordosis of cervical spine; B: Post-operative lateral fluoroscopy showing the ossification was hoisted and fused to an antedisplaced location, fixed fusion was conducted on the level C3/4 with a zero profile fusion device |
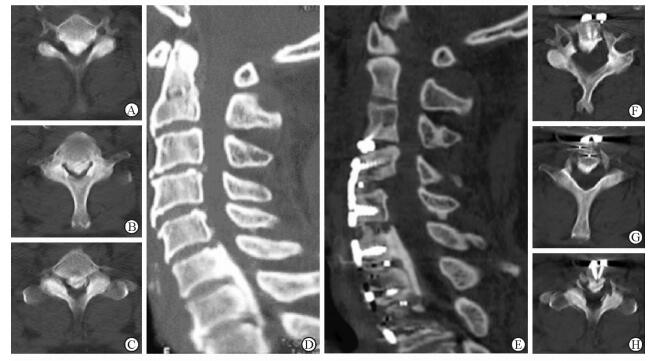
|
图 6 病例2术前及术后颈椎CT三维重建对比 Fig 6 Comparison of pre-and post-operative CT 3D reconstruction of case 2 A, F: C6 level of axial plane; B, G: C7 level of axial plane; C, H: C7 inferior endplate level of axial plane; D, E: Central sagittal plane. A-D: Pre-operative sagittal CT image showing osteophyte formation at the level C3/4 space, and a massive continuous type OPLL at C6-T1, canal narrowing rate was 70%, axial CT showing the ossification mass was located on the middle, causing severe canal narrowing; E-H: Post-operative sagittal CT image showing the ossification was hoisted and fused to an antedisplaced location, the spine canal narrowing rate was 5%. OPLL: Ossification of the posterior longitudinal ligament |

|
图 7 病例2术前及术后颈椎磁共振成像对比 Fig 7 Comparison of pre-and post-operative magnetic resonance imaging of case 2 A, F: C6 level of axial plane; B, G: C7 pedicel level of axial plane; C, H: T1 pedicel level of axial plane; D, E: Central sagittal plane. A-D: Pre-operative magnetic resonance imaging (MRI) showing severe compression caused by disc herniation at the level C3/4, C4/5 and C5/6 space, and it was caused by OPLL at the level C6-T1; E-H: Post-operative MRI showing the decompression of cervical spinal cord in each level, the signal of cerebral spinal fluid was observed in front of spinal cord at the level C4-T1, and the spinal dura was hoisted together with the OPLL because of the adhesion between them. OPLL: Ossification of the posterior longitudinal ligament |
3 讨论
OPLL病程较长,骨化进程缓慢,脊髓对于此类缓慢形成的压迫耐受性较强,故OPLL发病时骨化物往往较大。对于骨化物较大的OPLL,目前手术治疗策略尚无共识。颈椎后路减压手术通过切除椎板或椎板成形扩大颈椎管容积,使脊髓后移,实现神经的间接减压。后路手术难度较低,对于前路手术难以重建的长节段OPLL具有独特的优势[7]。但该术式的效果依赖脊髓的后移,故骨化物巨大及颈椎前凸较差时后路手术效果并不理想[8]。通常认为,椎管狭窄率大于60%的严重OPLL采用后路椎管扩大间接减压治疗效果不佳[5]。而前路减压手术通过从前方切除骨化物实现神经减压,其减压效果直接,但切除骨化物时操作难度较高,手术风险较大[9-10]。
传统前路手术主要为前路椎体次全切除术(anterior cervical corpectomy and fusion, ACCF),需切除骨化物以达到充分减压,无论是分块切除、整块切除或磨薄漂浮等方法,均可能对脊髓造成损伤,损伤发生率可高达14%[11-12]。后路减压手术不直接切除骨化物,于后路脊髓外侧对椎板进行开槽或切除操作,相对安全,不易损伤脊髓,神经损伤的发生率较低[13]。ACAF手术虽是前路直接减压,但其操作区域位于脊髓外侧的钩椎关节附近,在解剖上降低了脊髓损伤的风险。统计目前临床病例,仅有极少数患者(4%,未发表数据)出现上肢的一过性肌力下降,可能与神经根的刺激有关。
传统的后路减压手术无论是椎管成形术还是椎板切除术,均为从后方扩大椎管,使脊髓向后膨起或退让,良好的减压效果依赖于良好的颈椎曲度、适当的骨化物大小和合适的减压范围。然而过度的脊髓后移常导致神经根牵拉,造成术后C5神经根病的发生[14]。ACCF的减压是对骨化物的直接切除,但其减压范围通常为12~14 mm,对于基底部较宽的OPLL,常难以充分切除,遗留残余症状。ACAF开槽及椎间隙的减压宽度达到18~20 mm,解剖上此宽度超过了脊髓的宽度[15],达到神经根出口的内缘。故ACAF的减压是对脊髓和神经根的同时减压,较为充分。
OPLL前路手术的重要并发症之一是硬脊膜、蛛网膜破裂导致的术后脑脊液漏[16]。传统ACCF手术需切除与硬脊膜紧密相贴的后纵韧带,往往因为粘连或用钳方式不对造成硬脊膜卷入钳口,出现硬脊膜破裂;更为难以避免的是,当出现硬脊膜骨化时,传统ACCF切除骨化物后将可能出现大片硬脊膜缺损,术后脑脊液漏持续时间较长,影响术后恢复[17]。ACAF手术的优势在于不需直接处理后纵韧带和硬脊膜骨化之间的关系,而采用自两侧无骨化或骨化较轻处进行游离切断操作。在骨化物宽度较小的病例中,两侧开槽处可不切断后纵韧带,减少了脑脊液漏发生的风险。如ACAF术中出现脑脊液漏,可使用自体筋膜或人工硬脊膜覆盖材料进行覆盖,由于椎体骨化物复合物的存在,固定硬脊膜覆盖材料变得简单而有效。
ACCF术中出血相比后路手术较多,由于骨化物的压迫造成椎管内静脉回流受阻,使椎管内静脉丛和后纵韧带上的静脉处于怒张充盈状态,ACCF术中触及这些血管时常导致难以控制的出血,由于这些血管位于椎管两侧,间接导致ACCF两侧的减压存在顾虑而引起减压不充分[18]。ACAF手术在开槽时使用超声骨刀或高速磨钻,两者对骨面渗血都有一定的止血作用,骨面较多的出血可通过涂抹骨蜡止血。后纵韧带及椎管内静脉的出血有时较汹涌,但由于椎体骨化物复合物的存在,可使用骨蜡或止血材料进行压迫继而轻松实现止血。由于硬脊膜常与骨化物之间存在粘连,故骨化物被提拉后,硬脊膜被共同提起,形成ACAF术特殊的“帐篷效应”,故此硬脊膜外不存在血肿形成的空间,发生术后硬脊膜外血肿的可能性较小。
ACAF是一种通过前移椎管前壁而直接扩大椎管容积的方法,故前方压迫导致的颈椎管狭窄都是其适应证,对于OPLL患者,脊髓和神经根前方的压迫可通过ACAF直接减压。由于不直接切除骨化物,故椎管狭窄率的大小对手术难度的影响较小,而骨化物的宽度对手术难度的影响较大。如果骨化物有一个伸至椎管边缘的宽大基底,或骨化物整体位于椎管一侧,则因开槽难度大而视为相对禁忌。另一个相对禁忌是OPLL压迫节段过长的情况(≥5个节段),因目前尚没有足够长度的颈前路钛板可供重建。
颈椎前凸的存在与否与本手术的方案制定密切相关,如前凸曲度较差,则可利用预弯钛板的曲度对中间椎体起到较好的提拉效果;如前凸曲度较好,则需切除足够的椎体前部骨质,以达到足够的前移空间。对于骨化头尾两端的提拉因较难利用曲度调整,故骨化较大时需注意切除足够椎体前部骨质。
ACAF手术在设计上结合了传统颈椎前路手术减压直接彻底和后路手术操作安全的特点,初步临床结果证实了其治疗严重颈椎OPLL的可行性。虽然手术的安全性、有效性、并发症的发生率、植骨融合率、前移的骨化物是否进展等问题仍需进一步研究,但该创新术式所运用的独特思维方法将给OPLL的治疗策略带来全新的方向。
| [1] | HORIKOSHI T, MAEDA K, KAWAGUCHI Y, CHIBA K, MORI K, KOSHIZUKA Y, et al. A large-scale genetic association study of ossification of the posterior longitudinal ligament of the spine[J]. Hum Genet, 2006, 119: 611–616. DOI: 10.1007/s00439-006-0170-9 |
| [2] | KALB S, MARTIROSYAN N L, PEREZ-ORRIBO L, KALANI M Y, THEODORE N. Analysis of demographics, risk factors, clinical presentation, and surgical treatment modalities for the ossified posterior longitudinal ligament[J]. Neurosurg Focus, 2011, 30: E11. DOI: 10.3171/2010.12.FOCUS10265 |
| [3] | CHEN Y, GUO Y, LU X, CHEN D, SONG D, SHI J, et al. Surgical strategy for multilevel severe ossification of posterior longitudinal ligament in the cervical spine[J]. J Spinal Disord Tech, 2011, 24: 24–30. DOI: 10.1097/BSD.0b013e3181c7e91e |
| [4] | IWASAKI M, OKUDA S, MIYAUCHI A, SAKAURA H, MUKAI Y, YONENOBU K, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament:part 1:clinical results and limitations of laminoplasty[J]. Spine (Phila Pa 1976), 2007, 32: 647–653. DOI: 10.1097/01.brs.0000257560.91147.86 |
| [5] | FUJIMORI T, IWASAKI M, OKUDA S, TAKENAKA S, KASHⅡ M, KAITO T, et al. Long-term results of cervical myelopathy due to ossification of the posterior longitudinal ligament with an occupying ratio of 60% or more[J]. Spine (Phila Pa 1976), 2014, 39: 58–67. DOI: 10.1097/BRS.0000000000000054 |
| [6] | CHEN Y, CHEN D, WANG X, LU X, GUO Y, HE Z, et al. Anterior corpectomy and fusion for severe ossification of posterior longitudinal ligament in the cervical spine[J]. Int Orthop, 2009, 33: 477–482. DOI: 10.1007/s00264-008-0542-y |
| [7] | IWASAKI M, OKUDA S, MIYAUCHI A, SAKAURA H, MUKAI Y, YONENOBU K, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament:part 2:advantages of anterior decompression and fusion over laminoplasty[J]. Spine (Phila Pa 1976), 2007, 32: 654–660. DOI: 10.1097/01.brs.0000257566.91177.cb |
| [8] | SHIN J H, STEINMETZ M P, BENZEL E C, KRISHNANEY A A. Dorsal versus ventral surgery for cervical ossification of the posterior longitudinal ligament:considerations for approach selection and review of surgical outcomes[J]. Neurosurg Focus, 2011, 30: E8. DOI: 10.3171/2010.12.FOCUS10270 |
| [9] | BABA H, FURUSAWA N, TANAKA Y, WADA M, IMURA S, TOMITA K. Anterior decompression and fusion for cervical myeloradiculopathy secondary to ossification of the posterior ligament[J]. Int Orthop, 2000, 18: 204–209. |
| [10] | WANG X, CHEN D, YUAN W, ZHANG Y, XIAO J, ZHAO J. Anterior surgery in selective patients with massive ossification of posterior longitudinal ligament of cervical spine:technical note[J]. Eur Spine J, 2012, 21: 314–321. DOI: 10.1007/s00586-011-1996-y |
| [11] | KIM B, YOON D H, SHIN H C, KIM K N, YI S, SHIN D A, et al. Surgical outcome and prognostic factors of anterior decompression and fusion for cervical compressive myelopathy due to ossification of the posterior longitudinal ligament[J]. Spine J, 2015, 15: 875–884. DOI: 10.1016/j.spinee.2015.01.028 |
| [12] | MIZUNO J, NAKAGAWA H. Outcome analysis of anterior decompressive surgery and fusion for cervical ossification of the posterior longitudinal ligament:report of 107 cases and review of the literature[J]. Neurosurg Focus, 2001, 10: E6. DOI: 10.3171/foc.2001.10.4.7 |
| [13] | LI H, DAI L Y. A systematic review of complications in cervical spine surgery for ossification of the posterior longitudinal ligament[J]. Spine J, 2011, 11: 1049–1057. DOI: 10.1016/j.spinee.2011.09.008 |
| [14] | SAKAURA H, HOSONO N, MUKAI Y, ISHⅡ T, YOSHIKAWA H. C5 palsy after decompression surgery for cervical myelopathy:review of the literature[J]. Spine (Phila Pa 1976), 2003, 28: 2447–2451. DOI: 10.1097/01.BRS.0000090833.96168.3F |
| [15] | PETTY P. Surgical anatomy of the anterior cervical spine:the disc space, vertebral artery, and associated bony structures[J]. Neurosurgery, 1997, 41: 325. DOI: 10.1097/00006123-199707000-00072 |
| [16] | HANNALLAH D, LEE J, KHAN M, DONALDSON W F, KANG J D. Cerebrospinal fluid leaks following cervical spine surgery[J]. J Bone Joint Surg Am, 2008, 90: 1101–1105. DOI: 10.2106/JBJS.F.01114 |
| [17] | TIAN Y, YU K Y, WANG Y P, QIAN J, QIU G X. Management of cerebrospinal fluid leakage following cervical spine surgery[J]. Chin Med Sci J, 2008, 23: 121–125. DOI: 10.1016/S1001-9294(09)60024-9 |
| [18] | LIU X, WANG H, ZHOU Z, JIN A. Anterior decompression and fusion versus posterior laminoplasty for multilevel cervical compressive myelopathy[J]. Orthopedics, 2014, 37: e117–e122. DOI: 10.3928/01477447-20140124-12 |
 2017, Vol.
2017, Vol. 


