2. 重庆医科大学附属第一医院实验研究中心, 重庆 400016
2. Experimental Research Center, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
脑源性神经营养因子(brain-derived neurotrophic factor,BDNF)和胶质细胞源性神经营养因子(glial cell-derived neurotrophic factor,GDNF)均为体内重要的神经营养因子,广泛分布于前额叶皮质和海马等。前额叶皮质被认为是主要的攻击和暴力抑制中枢,局部直接刺激海马也可抑制攻击行为,海马还可投射谷氨酸能神经纤维到杏仁核、下丘脑背内侧核等调节攻击行为。多项研究显示攻击行为与下降的BDNF水平有关[1-3]。GDNF与攻击行为的研究多集中在与攻击行为密切相关的精神疾病中:有研究显示患者血浆GDNF水平升高或降低[4-5],结论并不一致;少量动物实验则发现G毒毛旋花苷所致躁狂模型大鼠前额叶皮质、海马内GDNF水平均降低[6];最新研究表明GDNF参与恐惧引起的攻击行为[7]。此外,BDNF、GDNF与5-羟色胺(serotonin, 5-HT)系统有着相互促进的作[8-9],而5-HT系统被认为是调控攻击行为的主要物质,尤其5-HT1A受体激动剂可以抑制大鼠攻击行为。
上述既往研究表明BDNF参与了攻击行为的生物学过程,本课题组前期研究也显示BDNF参与了早年慢性应激所致的病理性攻击(pathological aggression)行为的发生[2-3]。同为神经营养因子的GDNF是否有类似的作用,目前尚未见报道。因此,本研究通过比较5-HT1A受体激动剂干预前后青春期病理性攻击大鼠的攻击行为和前额叶皮质及海马内BDNF、GDNF蛋白表达,探讨该药物对病理性攻击行为和BDNF、GDNF表达的影响,为进一步探索其生物学机制提供依据。
1 材料和方法 1.1 实验动物及材料出生后21 d (P21) 的雄性SD大鼠56只,体质量50~70 g,清洁级,购于重庆医科大学实验动物中心[实验动物生产许可证号:SCXK(渝)2012-0001;使用许可证号:SYXK(渝)2012-0001]。所有动物随机分为6组:正常组、模型组、模型+药物组、模型+生理盐水(normal saline, NS)组、正常+药物组、正常+NS组,每组7只,余14只作为居住-入侵实验的工具鼠(入侵鼠)。本研究获得重庆医科大学附属第一医院生物医学伦理委员会批准。
5-HT1A受体选择性激动剂8-OH-DPAT购于美国Sigma公司,BDNF(ab101748) 和GDNF(ab176564) 抗体均购于英国Abcam公司,行为学摄像机为日本Sony公司产品。
1.2 建模方法模型组、模型+药物组、模型+NS组大鼠接受早年慢性应激,具体方法如下述,余3组及入侵鼠于大笼中正常喂养(4只/笼)。所有大鼠均自由饮食,维持标准温度和湿度。
1.2.1 早年孤养及昼夜颠倒应激自断奶后P21起单独小笼喂养,并予昼夜颠倒应激(8:00~20:00遮光,20:00~次日8:00光照),持续5周。
1.2.2 去奖赏挫败自P28起另予2%蔗糖水,自P35起,将糖水浓度减半至1%,并于每次居住-入侵实验前2 h撤走糖水,饮食自由,共3周。
1.2.3 预激惹刺激及居住-入侵实验从P35起,每日18:00将入侵鼠单独放入居住鼠笼内,于笼子正中用一铁丝网将入侵鼠与居住鼠隔开,预激惹居住鼠5 min,随即取出铁丝网,行1次10 min的居住-入侵实验,1次/d,共3周。
1.3 行为学测试(居住-入侵实验)5周后对6组大鼠进行1次10 min的居住-入侵实验,记录其攻击行为,由2名观察者观看录像后评估(盲法评定),取均值。与正常组比较有以下情况之一可视为动物有病理性攻击行为:(1) 攻击潜伏期显著缩短;(2) 攻击/威胁比例显著升高;(3) 攻击危险部位(头、腹和喉部);(4) 在居住鼠屈服后继续攻击;(5) 出现严重的攻击后果(出血、受伤或死亡);(6) 忽视对手状况(活动/静止/死亡)和环境(居住笼/中性环境)进行攻击[10]。
1.4 药物干预建模成功2周后,分别对模型+药物组、模型+NS组、正常+药物组、正常+NS组大鼠行腹腔注射8-OH-DPAT(0.5 mg/kg)或NS(2 mL/只),1次/d,持续2周(即P63~P77) 后对6组大鼠进行居住-入侵实验(方法同前)。
1.5 脑组织标本采集6组大鼠用10%水合氯醛按0.3 mL/100 g行腹腔注射麻醉加高浓度二氧化碳窒息后断头,参考《大鼠脑立体定位图谱》分离大脑前额叶皮质、海马,NS冲洗,-80 ℃保存。
1.6 蛋白质印迹法分析前额叶皮质、海马内BDNF及GDNF蛋白表达水平取大鼠前额叶皮质和海马组织进行裂解、匀浆,取上清液。蛋白定量后行聚丙烯酰胺凝胶电泳、电转膜,封闭(5%脱脂奶粉)后加入BDNF(1:500)、GDNF(1:4 000) 抗体孵育,4 ℃过夜。复温,洗膜(10 min/次,共3次),再加入辣根过氧化酶标记的山羊抗兔IgG二抗孵育, 37 ℃温箱放置2 h, 洗膜(10 min/次,共3次),用凝胶成像系统显影并分析结果。
1.7 统计学处理应用SPSS 21.0、GraphPad Prism 5软件进行分析,数据均以x±s表示,组间比较采用独立样本t检验或单因素方差分析。检验水准(α)为0.05。
2 结果 2.1 建模成功后青春期大鼠攻击行为检测与正常组比较,模型组大鼠攻击潜伏期缩短,差异有统计学意义(P=0.010);攻击持续时间、攻击总数均增加,差异有统计学意义(P < 0.01)。见表 1。
|
|
表 1 模型组与正常组大鼠攻击行为比较 Tab 1 Comparison of aggressive behaviors of rats in model and normal groups |
2.2 建模成功后青春期大鼠前额叶皮质及海马内
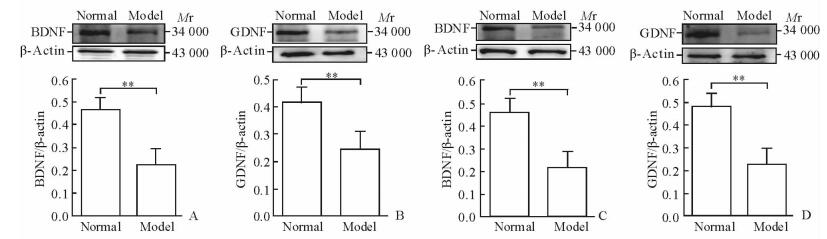
BDNF、GDNF蛋白表达蛋白质印迹分析结果显示模型组前额叶皮质及海马内BDNF、GDNF蛋白表达水平均低于正常组,差异有统计学意义(P < 0.01)。见图 1。
|
图 1 蛋白质印迹法检测模型组与正常组大鼠前额叶皮质及海马内BDNF、GDNF蛋白表达 Fig 1 Expressions of BDNF and GDNF in prefrontal cortex and hippocampus of rats in model and normal groups by Western blotting A, B: The relative protein expressions of BDNF and GDNF in prefrontal cortex, respectively; C, D: The relative protein expressions of BDNF and GDNF in hippocampus, respectively. BDNF: Brain-derived neurotrophic factor; GDNF: Glial cell-derived neurotrophic factor. **P < 0.01. n=7, x±s |
2.3 干预后青春期大鼠攻击行为检测
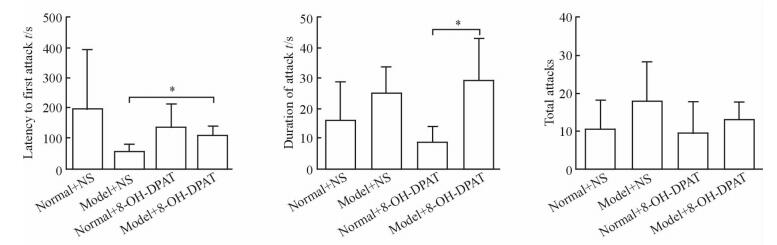
药物干预后,模型+药物组攻击潜伏期较模型+NS组延长(P < 0.05);模型+药物组攻击持续时间较正常+药物组仍增加(P < 0.05);攻击总数组间比较差异无统计学意义(P>0.05)。见图 2。
|
图 2 干预后4组大鼠的攻击行为检测结果 Fig 2 Aggressive behaviors of rats in four groups after intervention NS: Normal saline; 8-OH-DPAT: 8-Hydroxy-2-(di-n-propylamino)tetralin. *P < 0.05. n=7, x±s |
2.4 干预后青春期大鼠脑前额叶皮质及海马内BDNF、GDNF蛋白表达
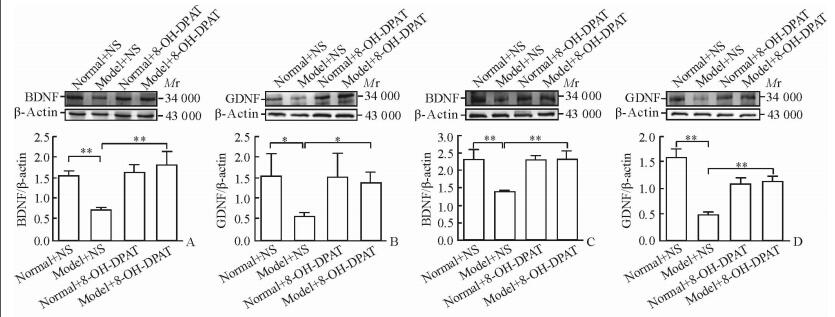
药物干预后,蛋白质印迹分析检测大鼠前额叶皮质及海马内BDNF、GDNF蛋白表达水平发现,模型+NS组较正常+NS组降低(P < 0.05,P < 0.01),模型+药物组较模型+NS组升高(P < 0.05,P < 0.01)。见图 3。
|
图 3 干预后4组大鼠前额叶皮质及海马内BDNF、GDNF蛋白表达 Fig 3 Expressions of BDNF and GDNF in prefrontal cortex and hippocampus of rats in four groups after intervention A, B: The relative protein expressions of BDNF and GDNF in prefrontal cortex, respectively; C, D: The relative protein expressions of BDNF and GDNF in hippocampus, respectively. NS: Normal saline; 8-OH-DPAT: 8-Hydroxy-2-(di-n-propylamino)tetralin. * P < 0.05, **P < 0.01. n=7, x±s |
3 讨论
病理性攻击是指增强的、病理的和异常形式的攻击行为[11],由生物、社会、心理因素引起的心理障碍或心理疾病而产生的对他人、社会规范及物体的侵犯行为[12]。病理性攻击具有攻击潜伏期短、攻击的强度与引发的刺激不匹配和忽视屈服等社会信号的特点,社会危害性大,因此病理性攻击的相关基础研究对未来预防和干预十分重要。
本研究显示模型组大鼠总体攻击水平高于正常组,与我们前期研究[13]结果一致,也与既往研究结果类似:Krupina等[14]发现孤养大鼠攻击性增加,Karpova等[15]也发现社会剥夺可增加大鼠攻击行为。8-OH-DPAT干预后大鼠的攻击潜伏期显著延长,而对于攻击持续时间和攻击总数并未出现预期的明显下降,说明5-HT1A受体激动剂可在一定程度上降低攻击行为,与已有研究[16]结果一致。这可能与给药途径及药物作用部位、用药剂量等有关。Stein等[17]在雄性小鼠下边缘皮质微量注射5-HT1A受体激动剂未能降低攻击行为。De Almeida等[18]发现激活内侧隔区突触后5-HT1A受体可增加大鼠攻击行为。有研究报道5-HT1A受体激动剂通过激活中缝背核突触前自受体发挥抗攻击作用,而早年应激可致中缝背核5-HT1A突触前自受体脱敏,因此抗攻击作用可能不明显。另外在一项仓鼠的研究中证实腹腔注射8-OH-DPAT可剂量依赖性减少可卡因所致的攻击[19]。
本实验还显示模型组前额叶皮质及海马内BDNF蛋白水平均低于正常组,与他人研究[1]结果类似,也与本课题组前期研究[2-3]结果一致。在使用8-OH-DPAT干预后,模型+药物组前额叶皮质及海马的BDNF蛋白水平较模型+NS组均升高。Galter和Unsicker[20]也发现8-OH-DPAT可增加BDNF mRNA表达,可能与以下调节有关:5-HT通过5-HT1A自受体上调BDNF和激活受体酪氨酸激酶(TrKB),上调的BDNF和激活的TrKB又可促进5-HT亚型的成熟(直接调节);BDNF还可通过刺激星形胶质细胞分泌中枢神经特异蛋白(β-S100) 和少突胶质细胞产生髓鞘磷脂蛋白调控5-HT系统(间接调控)。最新研究显示恐惧引起的攻击大鼠前额叶皮质内GDNF mRNA降低,然而前额叶皮质内GDNF单体却未检测到[7],这与本实验结果不一致可能在于其所采用的建模方式及所建模型不同。另外有学者对一些与攻击密切相关的精神障碍进行了探索:Tunca等[5]发现服用抗精神疾病药物的精神分裂症患者血清中GDNF显著低于健康对照组, 双相情感障碍患者躁狂发作时血浆GDNF升高;也有动物研究显示躁狂模型大鼠前额叶、海马内GDNF水平均低于对照组[6], 这可能与使用抗精神病药物、精神疾病类型及所处疾病阶段等因素有关。此外有研究显示GDNF很难透过血脑屏障[21], 故脑组织中GDNF蛋白与血浆/血清中GDNF蛋白水平的相关性并不确定。本研究中,5-HT1A受体激动剂干预后攻击大鼠前额叶皮质及海马内GDNF蛋白均增加,虽然既往未见有关文献报道,不过有研究显示双相情感障碍患者使用抗精神病药物治疗8周后GDNF较前显著升高[22],氟西汀可增加抑郁症患者血浆GDNF蛋白水平[23]。我们前期研究发现5-HT1A受体激动剂可增加大鼠前额叶皮质内环磷腺苷效应元件结合蛋白(CREB)磷酸化水平[24],而CREB结合位点已经被证实位于GDNF基因启动子序列内[25],故推测5-HT1A受体激动剂可能通过磷酸化CREB增加青春期病理性攻击大鼠前额叶皮质及海马内GDNF基因转录进而上调GDNF蛋白水平,其具体机制有待进一步深入研究。
综上,前额叶皮质及海马内BDNF、GDNF表达可能与早年慢性应激所致青春期大鼠病理性攻击行为的发生有关,5-HT1A受体激动剂上调上述脑区BDNF、GDNF的蛋白表达,并在一定程度上减轻早年慢性应激所致青春期大鼠病理性攻击行为。本研究存在一定的局限性,仅关注部分脑区,未检测血液中BDNF、GDNF等,未来可结合5-HT1A受体拮抗剂、5-HT与BDNF、GDNF通路相关物质,从全脑、血液等多方面探索青春期病理性攻击行为发生、发展的病理生理机制,为青少年暴力攻击的预防和干预提供一定的理论基础。
志谢
衷心感谢重庆医科大学附属第一医院实验研究中心的技术指导和帮助!
| [1] | YOCHUM C, DOHERTY-LYON S, HOFFMAN C, HOSSAIN M M, ZELIKOFF J T, RICHARDSON J R. Prenatal cigarette smoke exposure causes hyperactivity and aggressive behavior:role of altered catecholamines and BDNF[J]. Exp Neurol, 2014, 254: 145–152. DOI: 10.1016/j.expneurol.2014.01.016 |
| [2] | 雷莉, 张艳, 胡华, 秦光成, 陈力学, 屈远. 早年慢性应激对青春期大鼠攻击行为及下丘脑腹内侧核和前额皮质脑源性神经营养因子表达的影响[J]. 第三军医大学学报, 2013, 35: 233–236. |
| [3] |
张艳, 雷莉, 胡华, 屈远, 吕小娟, 秦光成, 等. 早年慢性应激对青春期病理性攻击大鼠空间学习记忆及海马脑源性神经营养因子、5-羟色胺的影响[J]. 第二军医大学学报, 2013, 34: 1065-1069. ZHANG Y, LEI L, HU H, QU Y, LÜ X J, QIN G C, et al. Effect of early life chronic stress on spatial learning and memory and hippocampus brain-derived neurotrophic factor and 5-HT in puberty pathological aggression rats[J]. Acad J Sec Mil Med Univ, 2013, 34: 1065–1069. |
| [4] | SHIM S H, HWANGBO Y, YOON H J, KWON Y J, LEE H Y, HWANG J A, et al. Increased levels of plasma glial-derived neurotrophic factor in children with attention deficit hyperactivity disorder[J]. Nord J Psychiatry, 2015, 9: 1–6. |
| [5] | TUNCA Z, KIVIRCIK AKDEDE B, ÖZERDEM A, ALKIN T, POLAT S, CEYLAN D, et al. Diverse glial cell line-derived neurotrophic factor (GDNF) support between mania and schizophrenia:a comparative study in four major psychiatric disorders[J]. Eur Psychiatry, 2015, 30: 198–204. |
| [6] | VARELA R B, VALVASSORI S S, LOPES-BORGES J, MARIOT E, DAL-PONT G C, AMBONI R T, et al. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats[J]. J Psychiatr Res, 2015, 61: 114–121. DOI: 10.1016/j.jpsychires.2014.11.003 |
| [7] | ILCHIBAEVA T V, TSYBKO A S, KOZHEMYAKINA R V, POPOVA N K, NAUMENKO V S. Glial cell line-derived neurotrophic factor (GDNF) in genetically defined fear-induced aggression[J]. Eur J Neurosci, 2016, 44: 2467–2473. DOI: 10.1111/ejn.2016.44.issue-7 |
| [8] | DELTHEI T, GUIARD B P, CERDAN J, DAVID D J, TANAKA K F, REPÉRANT C, et al. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice[J]. Neuropharmcology, 2008, 55: 1006–1014. DOI: 10.1016/j.neuropharm.2008.08.001 |
| [9] | TSUCHIOKA M, TAKEBAYASHI M, HISAOKA K, MAEDA N, NAKATA Y. Serotonin (5-HT) induces glial cell line-derived neurotrophic factor (GDNF) mRNA expression via the transactivation of fibroblast growth factor receptor 2(FGFR2) in rat C6 glioma cells[J]. Neurochem, 2008, 106: 244–257. DOI: 10.1111/j.1471-4159.2008.05357.x |
| [10] | DE ALMEIDA R M, MICZEK K A. Aggression escalated by social instigation by discontinuation of reinforecement ("frustration") in mice:inhibition by anpirtoline:a 5-HT1B receptor agonist[J]. Neuropsychopharmcology, 2002, 27: 171–181. DOI: 10.1016/S0893-133X(02)00291-9 |
| [11] | MICZEK K A, FISH E W, DE BOLD J F. Neurosteroids, GABA receptors and escalated aggressive behavior[J]. Horm Behav, 2003, 44: 242–257. DOI: 10.1016/j.yhbeh.2003.04.002 |
| [12] | 唐平. 病理性攻击行为的心理基础及其哲学本质[J]. 医学与哲学, 2004, 25: 65–67. |
| [13] | 屈远, 雷莉, 秦光成, 陈力学, 胡华. 青春期病理性攻击动物模型的初步构建[J]. 中华行为医学与脑科学杂志, 2011, 20: 630–632. DOI: 10.3760/cma.j.issn.1674-6554.2011.07.019 |
| [14] | KRUPINA N A, KHLEBNIKOVA N N, ORLOVA I N. [Early social isolation increases aggression and impairs a short-term habituation in acoustic startle reflex in rats][J]. Patol Fiziol Eksp Ter, 2015, 59: 4–15. |
| [15] | KARPOVA I V, MIKHEEV V V, MARYSHEVA V V, BYCHKOV E R, PROSHIN S N. Oxytocin-induced changes in monoamine level in symmetric brain structures of isolated aggressive C57BL/6 mice[J]. Bull Exp Biol Med, 2016, 160: 605–609. DOI: 10.1007/s10517-016-3228-2 |
| [16] | DE BOER S F, NEWMAN-TANCREDI A. Anti-aggressive effects of the selective high-efficacy 'biased' 5-HT1A receptor agonists F15599 and F13714 in male WTG rats[J]. Psychopharmacology (Berl), 2016, 233: 937–947. DOI: 10.1007/s00213-015-4173-x |
| [17] | STEIN D J, MICZEK K A, LUCION A B, DE ALMEIDA R M. Aggression-reducing effects of F15599, a novel selective 5-HT1A receptor agonist, after microinjection into the ventral orbital prefrontal cortex, but not in infralimbic cortex in male mice[J]. Psychopharmacology (Berl), 2013, 230: 375–387. DOI: 10.1007/s00213-013-3164-z |
| [18] | DE ALMEIDA R M, LUCION A B. 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats[J]. Psychopharmacol(Berl), 1997, 134: 392–400. DOI: 10.1007/s002130050476 |
| [19] | KNYSHEVSKI I, RICCI L A, McCANN T E. Serotonin type-1A receptors modulate adolescent, cocaine-induced offensive aggression in hamsters[J]. Physiol Behav, 2005, 85: 167–176. DOI: 10.1016/j.physbeh.2005.03.020 |
| [20] | GALTER D, UNSICKER K. Sequential activation of the 5-HT1A serotonin receptor and TrkB induces the serotonergic neuronal phenotype[J]. Mol Cell Neurosci, 2000, 15: 446–455. DOI: 10.1006/mcne.2000.0841 |
| [21] | KASTIN A J, AKERSTROM V, PAN W. Glial cell line-derived neurotrophic factor does not enter normal mouse brain[J]. Neurosci Lett, 2003, 340: 239–241. DOI: 10.1016/S0304-3940(03)00007-7 |
| [22] | ZHANG X B, ZHANG Z J, SHA W W, XIE C M, XI G J, ZHOU H H, et al. Effect of treatment on serum glial cell line-derived neurotrophic factor in bipolar patients[J]. J Affect Disords, 2010, 126: 326–329. DOI: 10.1016/j.jad.2010.03.003 |
| [23] | SUN H, ZHAO H, MA C, BAO F, ZHANG J, WANG D H, et al. Effects of electroacupuncture on depression and the production of glial cell line-derived neurotrophic factor compared with fluoxetine:a randomized controlled pilot study[J]. J Altern Complement Med, 2013, 19: 733–739. DOI: 10.1089/acm.2011.0637 |
| [24] | 吕小娟. 5-HT1A受体激动剂对青春期大鼠病理性攻击行为及前额叶皮层ERK-CREB通路的影响[D]. 重庆: 重庆医科大学, 2015. |
| [25] | BAECKER P A, LEE W H, VERITY A N, EGLEN R M, JOHNSON R M. Characterization of a promoter for the hum an glial cell line-derived neurotrophic factor gene[J]. Brain Res Mol Brain Res, 1999, 69: 209–222. DOI: 10.1016/S0169-328X(99)00106-0 |
 2017, Vol. 38
2017, Vol. 38