2. 第二军医大学热带医学与公共卫生学系环境卫生学教研室, 上海 200433;
3. 上海体育学院运动科学学院, 上海 200438
2. Department of Environment Health, Faculty of Tropical Medicine and Public Health, Second Military Medical University, Shanghai 200433, China;
3. School of Exercise Science, Shanghai University of Sport, Shanghai 200438, China
睡眠剥夺(sleep deprivation,SD)是一种常见的社会现象。根据SD的时间和缓急程度,可将SD分为急性睡眠剥夺(acute sleep deprivation,ASD)和慢性睡眠剥夺(chronic sleep deprivation,CSD)[1]。相对于ASD,CSD对学习记忆和工作绩效的影响更为严重[2],但目前对CSD导致学习记忆障碍的分子机制研究却不及ASD深入。研究表明海马内的多巴胺及其受体在短期记忆和工作记忆的形成、储存和提取过程中发挥重要作用[3-4],我们前期研究发现多巴胺D1受体(dopamine D1 receptor,D1R)激动剂SKF38393能够改善大鼠空间学习记忆能力;此外,CSD可使海马超微结构损伤[5]。研究表明神经元细胞膜上的D1R被激活后,可通过蛋白激酶(PKA)、有丝分裂原活化蛋白激酶(MAPK)和磷酸肌醇3条通路上调cAMP反应元件结合蛋白(CREB),从而提高神经元的兴奋性[6]。但是目前仍不清楚D1R的激活能否改善CSD导致的海马超微结构的损伤,同时也不清楚在D1R激动剂改善CSD导致学习记忆障碍的过程中哪条信号通路对海马神经元发挥调控作用。本研究首先观察了D1R激动剂SKF38393对CSD导致海马超微结构损伤的影响,然后通过qPCR和蛋白质印迹法检测大鼠CSD后海马内D1R相关信号通路关键因子的表达,旨在阐明SKF38393究竟通过海马内的哪条信号通路发挥作用,为进一步探讨多巴胺对CSD过程中海马功能调控的神经化学机制提供理论依据。
1 材料和方法 1.1 实验动物及分组处理选取雄性Sprague Dawley大鼠35只[第二军医大学实验动物中心提供,动物生产许可证号:SCXK(沪)2013-2016],体质量160~180 g,分笼饲养,每笼5只,食物和水自由摄取。适应饲养1周后,剔除体质量最轻、负重游泳时间最短和Morris水迷宫实验90 s仍找不到平台的11只大鼠,剩余24只大鼠分为CSD组、SKF(CSD+注射激动剂SKF38393) 组和TC(大平台对照)组,每组8只。CSD组大鼠应用小动物睡眠剥夺箱[6]进行SD 18 h/d (16:00—次日10:00),放回笼内休息6 h/d (10:00—16:00),连续21 d;SKF组大鼠从CSD第15天开始,腹腔注射D1R激动剂SKF38393(1 mg/kg),连续7 d;TC组小动物睡眠剥夺箱内设有大平台,直径为18 cm,大鼠可在大平台上自由活动、睡眠,其他环境与CSD和SKF组相同。实验前适应训练3 d (2 h/d),实验过程中每2 d记录1次大鼠体质量。
1.2 透射电镜观察海马超微结构CSD 21 d后,每组随机选取2只大鼠,腹腔麻醉(10%水合氯醛,400 mg/kg),经升主动脉灌注4 ℃生理盐水冲洗血液,然后用含4%多聚甲醛、1.25%戊二醛的PBS灌注固定,在体视显微镜下选取海马的CA1区,环氧树脂包埋,超薄切片,枸橼酸铅和醋酸铀双染,透射电镜观察并采集图像。
1.3 qPCR检测海马内D1R相关信号通路关键因子mRNA表达每组剩余6只大鼠用水合氯醛麻醉后经升主动脉灌注4 ℃生理盐水冲洗血液,取出全脑,分离双侧海马,经液氮速冻后置于-80 ℃保存备用。取每只大鼠的一侧海马提取总RNA,测定浓度,分析纯度。以RNA为模板进行反转录获得cDNA,然后应用qPCR仪进行扩增(95 ℃ 5 s,60 ℃ 34 s;40个循环)。根据溶解曲线的值计算反转录产物的相对含量。引物序列见表 1。
|
|
表 1 多巴胺D1受体相关信号通路关键因子的引物序列 Tab 1 Primer sequences of key molecules of dopamine D1 receptor related signal pathways |
1.4 蛋白质印迹法检测海马内D1R相关信号通路关键因子蛋白的表达
取另一侧海马标本提取总蛋白,BCA定量蛋白浓度为2.5 μg/μL,然后依次进行SDS-PAGE(每孔上样总蛋白40 μg)、转膜(PVDF膜)、封闭1 h,4 ℃过夜标记一抗:蛋白激酶A催化亚基α(PKAcα;1:1 000稀释,Abcam,ab76238)、磷酸化PKAcα(p-PKAcα,1:1 000稀释,CST,#5661)、细胞外调节蛋白激酶1/2(ERK1/2;1:1 000稀释,BioWorld,BS1112)、磷酸化ERK1/2(p-ERK1/2,1:1 000稀释,BioWorld,BS5016)、磷脂酶Cβ1 (PLCβ1;1:1 000稀释,Abcam,ab77743)、钙/钙调蛋白依赖性蛋白激酶Ⅳ(CaMKⅣ;1:1 000稀释,Abcam,ab75874)、磷酸化CaMKⅣ(p-CaMKⅣ;1:1 000稀释,Abcam,ab59424)、β肌动蛋白(β-actin;1:1 000,Santa Cruz,sc-1616r)。次日室温标记相应二抗1 h。使用凝胶成像仪进行ECL发光、采集图像和灰度值分析。
1.5 统计学处理采用SPSS 18.0软件进行数据分析。计量资料以x±s表示,组间比较采用单因素方差分析(one-way ANOVA)。检验水准(α)为0.05。
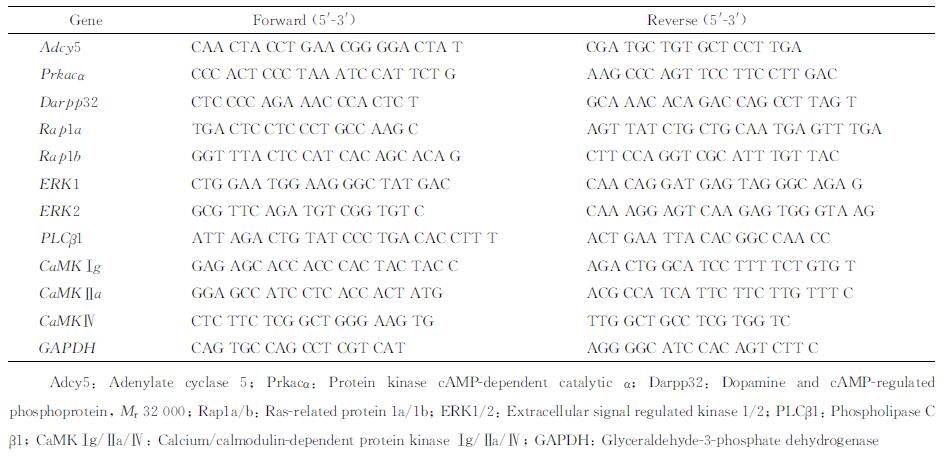
2 结果 2.1 D1R激动剂对CSD导致的海马超微结构损伤的改善作用透射电镜观察显示TC组大鼠海马神经元排列规则,染色质均匀,核仁清晰,细胞器丰富、结构完整、边缘清晰(图 1A)。CSD组大鼠海马神经元线粒体数量减少、肿胀甚至破裂,嵴结构模糊紊乱;部分神经元突触水肿、囊泡减少;突触后致密带较薄甚至出现结构不完整;粗面内质网结构模糊,其上的核糖体数量减少,排列凌乱(图 1B)。SKF组大鼠海马的超微结构虽不及TC组完好,但线粒体的肿胀程度、突触后致密带和各种膜结构较CSD组得到改善(图 1C)。
|
图 1 多巴胺D1受体激动剂SKF38393对CSD导致海马超微结构损伤的改善作用 Fig 1 Dopamine D1 receptor (D1R) agonist SKF38393 improved the ultrastructure of hippocampus injured by CSD A: TC group; B: CSD group; C: SKF group. TC: Tank control; CSD: Chronic sleep deprivation; SKF: CSD+D1R agonist SKF38393. Black arrows indicate mitochondria, and white arrows indicate postsynaptic dense bands. Scale bar=1 μm |
2.2 D1R激动剂对海马PKA信号通路关键因子表达的影响
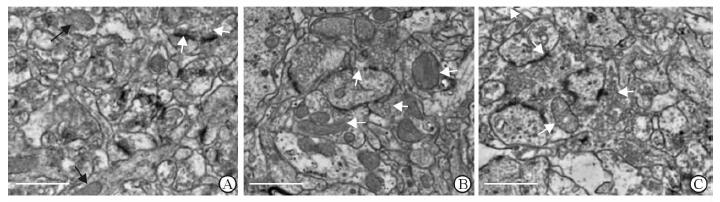
PKA信号通路关键因子的表达见图 2。与TC组相比,CSD组大鼠海马Adcy5、Prkacα和Darpp32 mRNA的表达均降低(P<0.05),PKAcα总蛋白及磷酸化表达均降低(P<0.05);SKF组的Adcy5 mRNA的表达也减少(P<0.05)。与CSD组相比,SKF组大鼠海马Prkacα、Darpp32 mRNA和PKAcα总蛋白及其磷酸化的表达均增加(P<0.05),而Adcy5 mRNA的表达差异无统计学意义(P>0.05)。
|
图 2 D1R激动剂SKF38393对海马PKA信号通路关键因子表达的影响 Fig 2 Effect of D1R agonist SKF38393 on key factor expression of PKA signaling pathway in the hippocampus of rats A-C: qPCR results; D, E: Western blotting results. TC: Tank control; CSD: Chronic sleep deprivation; SKF: CSD+D1R agonist SKF38393. D1R: Dopamine D1 receptor; Adcy5: Adenylate cyclase 5; Prkacα: Protein kinase cAMP-dependent catalytic α; Darpp32: Dopamine and cAMP-regulated phosphoprotein, Mr 32 000; PKAcα: Protein kinase A catalytic subunit α; p-PKAcα: Phosphorylated PKAcα. *P < 0.05. n=6, x±s |
2.3 D1R激动剂对海马MAPK信号通路关键因子表达的影响
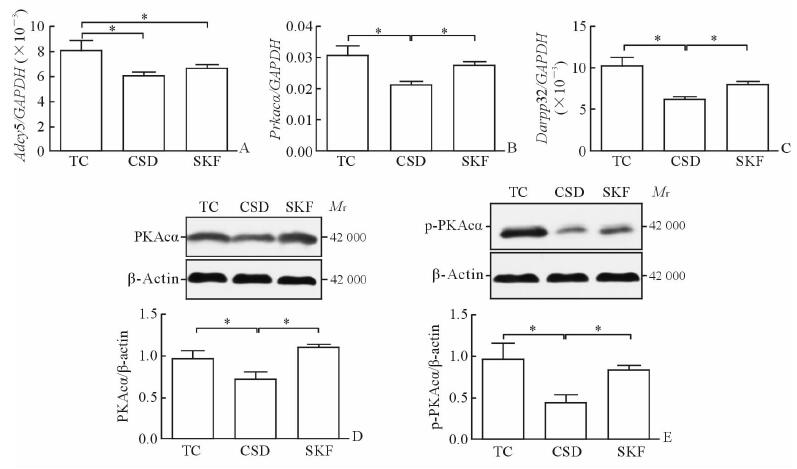
MAPK信号通路关键因子的表达见图 3。与TC组相比,CSD组大鼠海马内Rap1a、ERK1、ERK2 mRNA和ERK1/2蛋白磷酸化表达均下降(P<0.05),SKF组ERK2 mRNA和ERK1/2磷酸化的表达也降低(P<0.05)。但与CSD组相比,SKF组大鼠海马Rap1a、Rap1b和ERK1 mRNA的表达均增加(P<0.05);此外,ERK2 mRNA的表达上调,但与CSD组差异无统计学意义(P>0.05)。
|
图 3 D1R激动剂SKF38393对海马MAPK信号通路关键因子表达的影响 Fig 3 Effect of D1R agonist SKF38393 on key factor expression of MAPK signaling pathway in the hippocampus of rats A-D: qPCR results; E, F: Western blotting results. TC: Tank control; CSD: Chronic sleep deprivation; SKF: CSD+D1R Dagonist SKF38393. D1R: Dopamine D1 receptor; Rap1a/1b: Ras-related protein 1a/1b; ERK1/2: Extracellular signal regulated kinase 1/2; p-ERK1/2: Phosphorylated ERK1/2. *P < 0.05. n=6, x±s |
2.4 D1R激动剂对海马磷酸肌醇信号通路关键因子表达的影响
磷酸肌醇信号通路关键因子的表达见图 4。CSD组大鼠海马内PLCβ1、CaMKⅡa、CaMKⅣ mRNA和PLCβ1蛋白的表达均较TC组降低(P<0.05);SKF组的PLCβ1、CaMKⅡa mRNA和PLCβ1蛋白的表达均低于TC组(P<0.05),但与CSD组相比,SKF组大鼠海马内CaMKⅣ mRNA及其蛋白磷酸化表达均增加(P<0.05)。

|
图 4 D1R激动剂SKF38393对海马磷酸肌醇信号通路关键因子表达的影响 Fig 4 Effect of D1R agonist SKF38393 on key factor expression of phosphoinositol signaling pathway in the hippocampus of rats A-D: qPCR results; E-G: Western blotting results. TC: Tank control; CSD: Chronic sleep deprivation; SKF: CSD+D1R agonist SKF38393. D1R: Dopamine D1 receptor; PLCβ1: Phospholipaae C β1; CaMK Ⅰ g/Ⅱ a/Ⅳ: Calcium/calmodulin-dependent protein kinaseⅠ g/Ⅱ a/Ⅳ; p-CaMKⅣ: Phosphorylated CaMKⅣ. *P < 0.05. n=6, x±s |
3 讨论
本研究在前期工作[5]的基础上进一步证实D1R激动剂SKF38393可以改善CSD导致的海马超微结构损伤。激活D1R通过哪些下游信号通路发挥作用,是深入研究D1R激动剂的作用机制、探寻新型药物干预靶点的关键。D1R与其配体结合可激活腺苷酸环化酶(adenylyl cyclase,AC)催化产生cAMP,而SD可使海马中磷酸二酯酶(PDE)活性增加(主要为PDE4),导致第二信使cAMP含量下降[7],最终通过PKA、MAPK和磷酸肌醇3条信号通路降低神经元的兴奋性[8-10]。本研究结果显示,CSD组大鼠海马内Adcy5、Prkacα、Darpp32 mRNA和PKAcα总蛋白及其磷酸化的表达均较TC组降低;而给予D1R激动剂SKF38393后,Prkacα、Darpp32 mRNA和PKAcα总蛋白及其磷酸化的表达均较CSD组增加。可见CSD可通过影响大鼠海马内D1R相关的PKA信号通路使其发生损伤,而给予SKF38393干预可改善。
磷酸肌醇偶联多巴胺受体主要分布于额叶皮质和海马中,与学习记忆的调控密切相关。研究表明,磷酸肌醇偶联多巴胺受体激活可使PLCβ和位于内质网膜上三磷酸肌醇受体活化,促进钙释放,钙的释放使CaMKⅡ和CaMKⅣ活化,而CaMKⅡ和CaMKⅣ的活化又可使CREB活化,从而改善认知功能[11-12]。本研究结果表明在CSD过程中,海马内D1 R的磷酸肌醇通路也受到了严重损伤,且SKF38393的干预能够通过磷酸肌醇通路提高CREB磷酸化水平进而改善。
SD可使海马CREB磷酸化水平增加[10],或者通过Darpp32间接活化。本研究结果显示,CSD组大鼠海马内PLCβ1、CaMKⅡa、CaMKⅣ mRNA和PLCβ1蛋白的表达均较TC组降低,而给予SKF38393干预后大鼠海马内CaMKⅣ mRNA及其蛋白磷酸化表达均较CSD组增加。由此可见,MAPK信号通路在一定程度上参与了CSD导致的海马超微结构损伤。
本研究结果显示,CSD组大鼠海马内Rap1a、ERK1、ERK2 mRNA和ERK1/2蛋白磷酸化表达较TC组均下降,而给予SKF38393干预后,Rap1a、Rap1b和ERK1 mRNA的表达较CSD组均增加,且给予SKF38393干预后大鼠海马内ERK2 mRNA的表达有所上调(但与CSD组差异无统计学意义)。因此,MAPK信号通路可能参与了CSD导致的海马超微结构损伤,但作用较小。
| [1] | PHILIP P, SAGASPE P, PRAGUE M, TASSI P, CAPELLI A, BIOULAC B, et al. Acute versus chronic partial sleep deprivation in middle-aged people: differential effect on performance and sleepiness[J]. Sleep, 2012, 35: 997–1002. DOI: 10.5665/sleep.1968 |
| [2] | TASSI P, SCHIMCHOWITSCH S, ROHMER O, ELBAZ M, BONNEFOND A, SAGASPE P, et al. Effects of acute and chronic sleep deprivation on daytime alertness and cognitive performance of healthy snorers and non-snorers[J]. Sleep Med, 2012, 13: 29–35. DOI: 10.1016/j.sleep.2011.06.017 |
| [3] | SARIÑANA J, TONEGAWA S. Differentiation of forebrain and hippocampal dopamine 1-class receptors, D1R and D5R, in spatial learning and memory[J]. Hippocampus, 2016, 26: 76–86. DOI: 10.1002/hipo.22492 |
| [4] | SARIÑANA J, KITAMURA T, KÜNZLER P, SULTZMAN L, TONEGAWA S. Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory[J]. Proc Natl Acad Sci USA, 2014, 111: 8245–8250. DOI: 10.1073/pnas.1407395111 |
| [5] | WEN X S, CHEN X M, RONG F, JING T, CHEN S, MA W L. The regulation of SKF38393 on the Dopamine and D1 receptor expression in hippocampus during chronic REM sleep restriction[J]. CNS Neurosci Ther, 2013, 19: 730–733. DOI: 10.1111/cns.12140 |
| [6] | MAROUN M, AKIRAV I. Differential involvement of dopamine D1 receptor and MEK signaling pathway in the ventromedial prefrontal cortex in consolidation and reconsolidation of recognition memory[J]. Learn Mem, 2009, 16: 243–247. DOI: 10.1101/lm.1245009 |
| [7] | ROOSTERMAN D. Agonist-dependent and -independent dopamine-1-like receptor signalling differentially regulates downstream effectors[J]. FEBS J, 2014, 281: 4792–4804. DOI: 10.1111/febs.13018 |
| [8] | XING B, KONG H, MENG X, WEI S G, XU M, LI S B. Dopamine D1 but not receptor D3 is critical for spatial learning and related signaling in the hippocampus[J]. Neuroscience, 2010, 169: 1511–1519. DOI: 10.1016/j.neuroscience.2010.06.034 |
| [9] | WEN X, CHEN X, CHEN S, TAN Y, RONG F, ZHU J, et al. Influence of SKF38393 on changes of gene profile in rat prefrontal cortex during chronic paradoxical sleep deprivation[J]. Behav Brain Res, 2016, 304: 60–66. DOI: 10.1016/j.bbr.2016.02.002 |
| [10] | LEE K W, HONG J H, CHOI I Y, CHE Y, LEE J K, YANG S D, et al. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase[J]. J Neurosci, 2002, 22: 7931–7940. |
| [11] | GUAN Z, PENG X, FANG J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus[J]. Brain Res, 2004, 1018: 38–47. DOI: 10.1016/j.brainres.2004.05.032 |
| [12] | PANCHALINGAM S, UNDIE A S. SKF83959 exhibits biochemical agonism by stimulating [35 S] GTPγS binding and phosphoinositide hydrolysis in rat and monkey brain[J]. Neuropharmacology, 2001, 40: 826–837. DOI: 10.1016/S0028-3908(01)00011-9 |
 2017, Vol. 38
2017, Vol. 38