慢性肾脏病(chronic kidney disease,CKD)是全球重要的公共卫生问题之一。研究表明,30%~70%的CKD患者在疾病的进展过程中可出现蛋白质能量消耗不足(protein energy wasting,PEW),其最重要的特征是肌肉萎缩[1-3]。肌肉萎缩一旦发生,将严重影响CKD患者的临床预后及生活质量,同时增加心血管疾病的患病率[2]。想要攻克CKD导致肌肉萎缩这一难题,就需要探索其发生机制。有证据表明,线粒体功能异常可能参与CKD肌肉萎缩的发生[4-7],但具体机制尚不清楚。
Rho相关卷曲螺旋形成蛋白激酶1(Rho-associated coiled-coil containing protein kinase 1,ROCK1) 是RhoA激酶的下游活性调节分子,在应力纤维的形成、细胞坏死等方面发挥重要作用[8]。本课题组前期研究证实,ROCK1激活参与CKD患者肌肉萎缩的发展[9],其具体的机制尚未明确。但有证据表明,高糖诱导ROCK1激活,导致足细胞和肾小管上皮细胞中线粒体的裂变,并增加糖尿病鼠蛋白尿的发生,给予ROCK1的抑制剂法舒地尔(fasudil)可以阻断糖尿病鼠蛋白尿的进展[10]。因此,本研究通过腺病毒感染C2C12成肌细胞诱导ROCK1过表达,探讨ROCK1导致肌肉萎缩的发生是否与细胞呼吸功能异常及线粒体异常相关。
1 材料和方法 1.1 细胞培养与腺病毒感染体外培养C2C12成肌细胞(购自ATCC细胞库,货号CRL-177TM),培养于含10%胎牛血清(Gibco,美国,货号1082147),200 U/mL青霉素、50 μg/mL链霉素(Life Technologies)的DMEM培养液(HyClone)。当C2C12成肌细胞融合度达90%时,替换培养液为添加2%马血清(Gibco,新西兰,货号26050088)、200 U/mL青霉素、50 μg/mL链霉素的DMEM培养液,诱导C2C12成肌细胞分化成熟为肌小管细胞。将分化成熟的C2C12成肌细胞分为以下4组并给予不同处理:(1) Ad-GFP组,给予绿色荧光蛋白(GFP)腺病毒载体(AAV-GFP,2 nmol/L)感染细胞;(2) Ad-ROCK1组,给予ROCK1腺病毒载体(AAV-ROCK1,5 nmol/L)感染细胞;(3) Ad-GFPF组,给予2 nmol/L AAV-GFP感染同时用10 μmol/L法舒地尔刺激细胞;(4) Ad-ROCK1F组,给予5 nmol/L AVV-ROCK1感染同时用10 μmol/L法舒地尔刺激细胞。各组腺病毒感染或同时与法舒地尔共刺激均持续48 h。实验所用AVV-ROCK1、AVV-GFP由美国Dr.Chang实验室提供。
1.2 细胞能量代谢分析Seahorse是体外通过外通量分析精确检测细胞耗氧率(oxygen consumption rate,OCR)及胞外酸化率(extracellular acidification rate,ECAR)的方法,可一定程度地反映细胞呼吸情况。具体方法:(1) 利用胰酶消化C2C12成肌细胞,按2.5×104/孔接种到Seahorse测量专用细胞培养板(货号100777004,美国),待细胞融合度达90%时诱导分化成熟。按照前文描述的分组给予相应刺激。(2) 各组细胞完成不同的处理后进行上机测量,在测量前1 d,每孔均添加1 mL的Seahorse探头校准液进行水化,置于37 ℃、无CO2的温箱中过夜。(3) 按照线粒体应激(Mito-Stress)测定要求,使用相应培养液洗细胞后加入上机检测所需培养液,置于37 ℃、无CO2的温箱中45~60 min后,在Seahorse测量板A孔中加入寡霉素[oligomycin,是线粒体电子呼吸链中复合体Ⅴ-ATP合酶的抑制剂,可降低OCR],B孔中加入羰基-氰-对-三氟甲氧基苯腙[carbonyl cyanide-p(trifluoromethoxy) phenylhydrazone, FCCP;线粒体呼吸链解偶联剂,作用于线粒体内膜,去除线粒体内外膜之间的质子势能使OCR增加,但不产生ATP,使得能量均以热能的形式释放,产生大量热量],C孔中加入抗霉素A (antimycin A,线粒体电子呼吸链中复合体Ⅲ的抑制剂,可减少OCR)。(4) 将Seahorse测量板放在Seahorse测量仪上校准后,放入处理好的细胞培养板,设定程序为Mito-Stress,执行相应的步骤。检测结束后,按照所得数据计算细胞呼吸能力,其中基础呼吸是细胞在加寡霉素刺激之前的OCR值;最大呼吸是加入FCCP之后、抗霉素A之前OCR最大值与加入寡霉素之后OCR最低值之差;偶联ATP产生的呼吸是基础呼吸OCR值和加入FCCP刺激之前的OCR值之差。
1.3 蛋白质印迹法检测线粒体动力相关蛋白活性和肌肉萎缩相关蛋白的表达使用添加有蛋白酶和磷酸酶抑制剂(Thermo Fisher)的RIPA裂解和提取液(G-Biosciences,货号786-490) 提取C2C12成肌细胞蛋白,使用BCA蛋白测定试剂盒(Thermo Scientific)测定蛋白质浓度。如前期研究[9]所述方法进行蛋白质印迹实验。使用抗体ROCK1(CST,货号4035S,稀释比例1:1 000)、动力相关蛋白1(dynamin-related protein 1,Drp1;CST,货号8570,稀释比例1:1 000)、磷酸化Drp1(p-Drp1;CST,货号3455S,稀释比例1:1 000)、E3泛素连接酶肌肉环指蛋白1(muscle RING finger-1,MuRF1;Santa Cruz,货号sc-398608,稀释比例1:500)、肌肉萎缩F-box(muscle atrophy F-box,MAFbx,又称Atrogin1[11];Santa Cruz,货号sc-121485,稀释比例1:100)、β-肌动蛋白(β-actin;CST,货号3700,稀释比例1:1 000)。
1.4 细胞线粒体染色实验过程中,使用线粒体形态特异性染色试剂MitoTracker® Red CMXRos红色荧光探针(Thermo Scientific,货号M7512) 进行线粒体形态染色。实验步骤按照试剂说明书进行操作。
1.5 统计学处理应用SPSS 18.0软件进行数据分析。实验所得数据均以x±s表示,两组间比较采用t检验,多组样本比较采用单因素方差分析(one-way ANOVA),两两比较采用LSD-t检验。检验水准(α)为0.05。
2 结果 2.1 ROCK1过表达引起C2C12成肌细胞呼吸异常根据Seahorse中Mito-Stress结果(图 1),ROCK1过表达引起C2C12成肌细胞OCR和ECAR增加。ROCK1过表达能够增加C2C12成肌细胞的基础呼吸(P<0.01)、细胞最大呼吸(P<0.01) 及电子呼吸链偶联参与产生ATP的呼吸(P<0.01)。
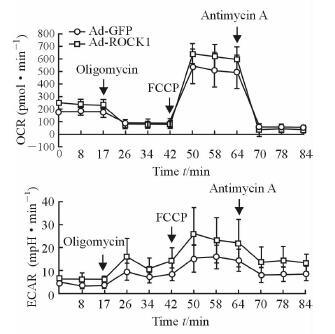
|
图 1 ROCK1过表达对C2C12成肌细胞呼吸功能的影响 Fig 1 Effect of ROCK1 overexpression on respiratory functions of C2C12 myoblasts Seahorse Mito-Stress results. OCR: Oxygen consumption rate; ECAR: Extracellular acidification rate; ROCK1: Rho-associated coiled-coil containing protein kinase 1; GFP: Green fluorescent protein; FCCP: Carbonyl cyanide-p (trifluoromethoxy) phenylhydrazone. n=6, x±s |
2.2 ROCK1过表达导致C2C12成肌细胞线粒体裂变增加
使用MitoTracker® Red MXRos红色荧光探针对C2C12成肌细胞进行染色,结果显示,与Ad-GFP组相比,ROCK1过表达可使C2C12成肌细胞线粒体裂变增加,体积变小,线粒体大小频数分布左移(图 2)。
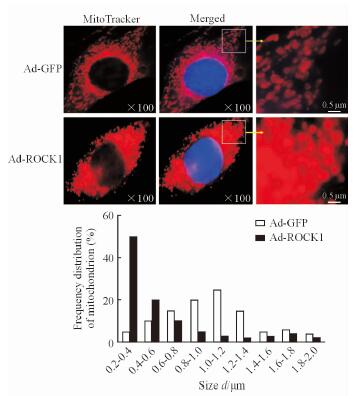
|
图 2 ROCK1过表达对C2C12成肌细胞线粒体形态的影响 Fig 2 Effect of ROCK1 overexpression on mitochondrial morphology of C2C12 myoblasts MitoTracker staining results showed the mitochondrial fragment number in Ad-ROCK1 group was increased, and the frequency distribution of mitochondrial size shifted to the left in comparison to Ad-GFP group. ROCK1: Rho-associated coiled-coil containing protein kinase 1; GFP: Green fluorescent protein |
2.3 法舒地尔改善ROCK1过表达导致的C2C12成肌细胞呼吸异常
Seahorse Mito-stress结果(图 3)示:法舒地尔在一定程度上降低了ROCK1过表达时C2C12成肌细胞在Mito-stress过程中增加的OCR和EACR;同时,法舒地尔能够减弱ROCK1过表达导致的基础呼吸、最大呼吸的增加程度,差异具有统计学意义(P<0.05)。Ad-ROCK1F和Ad-GFPF组相比细胞基础呼吸和最大呼吸无明显改变(P>0.05)。
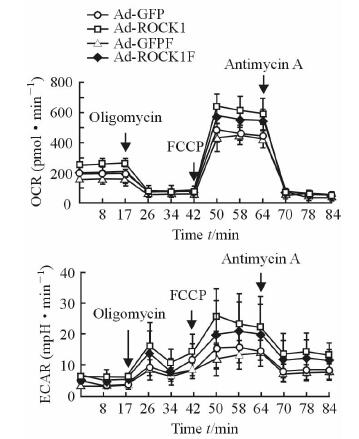
|
图 3 法舒地尔改善ROCK1过表达导致的C2C12成肌细胞呼吸异常 Fig 3 Fasudil reducing respiratory dysfunction of C2C12 myoblasts caused by ROCK1 overexpression Seahorse Mito-Stress results. OCR: Oxygen consumption rate; ECAR: Extracellular acidification rate; ROCK1: Rho-associated coiled-coil containing protein kinase 1; GFP: Green fluorescent protein; FCCP: Carbonyl cyanide-p (trifluoromethoxy) phenylhydrazone. n=6, x±s |
2.4 法舒地尔对线粒体动力相关蛋白活性和肌肉萎缩相关蛋白表达的影响
蛋白质印迹分析结果显示,法舒地尔能够降低ROCK1过表达引起的促进线粒体裂变的Drp1激活(p-Drp1/Drp1增加),以及ROCK1过表达引起的肌肉萎缩相关蛋白MuRF1和Atrogin1表达的增加(图 4)。
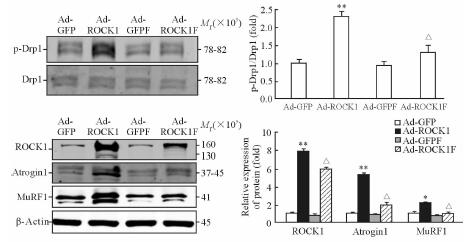
|
图 4 法舒地尔对线粒体动力相关蛋白活性和肌肉萎缩相关蛋白表达的影响 Fig 4 Effect of fasudil stimulation on activity of mitochondrial dynamin-related protein and expression of muscle atrophy-related proteins Western blotting results. ROCK1: Rho-associated coiled-coil containing protein kinase 1; GFP: Green fluorescent protein; Drp1: Dynamin-related protein 1; p-Drp1: Phosphorylated Drp1; Atrogin1: MAFbx, Muscle atrophy F-box; MuRF1: Muscle RING finger-1. *P < 0.05, **P < 0.01 vs Ad-GFP group; △P < 0.05 vs Ad-ROCK1 group. n=3, x±s |
3 讨论
CKD时机体蛋白合成减少、蛋白降解增加导致了肌肉萎缩的发生[12-16]。体内蛋白的降解主要依靠激活ATP-依赖的泛素蛋白酶系统[12],这一系统的激活需要消耗大量能量。线粒体作为机体能量产生的主要场所,其形态、数量及细胞内分布都反映了机体的代谢状态。生理状态下, 线粒体裂变和聚变维持相对平衡,共同抵抗外界的有害刺激,这一平衡一旦被打破,就会导致线粒体发生功能紊乱,进而导致机体能量供应异常[17]。
本实验为探究ROCK1过表达的C2C12成肌细胞是否存在线粒体功能异常,通过Seahorse检测细胞呼吸。首先测定OCR,在加入寡霉素之前显示的OCR值代表细胞的基础耗氧率,可认为是基础呼吸,主要包括线粒体氧化磷酸化及质子漏所消耗的氧(即质子经线粒体膜上的呼吸链传递形成一侧高电势,趋使一部分质子回流,通过ATP合酶形成ATP,将势能转化为ATP能量;另一部分质子通过线粒体膜后只是发生氧化,将势能转化为热量,并没有用于合成ATP)。寡霉素是ATP合酶抑制剂,加入后,OCR降低值代表机体用于ATP合成的耗氧量,间接反映细胞此时的ATP产量。实验中发现,ROCK1过表达不仅增加细胞的基础呼吸,而且增加细胞ATP的产生,但这种ATP的增加以缩短细胞寿命为代价(Seahorse检测结束后发现ROCK1过表达组的细胞大量死亡)。FCCP是一种线粒体电子呼吸链解偶联剂,其作为一种质子载体,可以使大量质子回流,大量耗氧,但这种质子回流不能产生ATP。加入FCCP后OCR的增加值代表线粒体的最大耗氧能力,间接反映最大的呼吸能力,而其与基础呼吸的差值代表细胞的呼吸潜力。本实验中,ROCK1过表达组细胞最大呼吸能力增加。最后加入的抗霉素A,其作为呼吸链抑制剂,完全阻止线粒体耗氧。因此,结合Seahorse结果,可认为ROCK1过表达能够导致细胞基础呼吸、最大呼吸及ATP的产生增加,但在一定程度上,这种高代谢的呼吸消耗加速了细胞的死亡。为进一步验证实验结果,我们对各组细胞线粒体进行形态和功能的特异性染色,结果表明,ROCK1过表达后细胞线粒体裂变增加,呈现碎片化,线粒体大小频数分布左移。
法舒地尔作为ROCK1抑制剂,目前临床上用于改善心脑血管循环,但并没有应用于改善肌肉萎缩的报道。我们的研究结果显示,法舒地尔可在一定程度上改善ROCK1过表达后造成细胞高耗氧的状态,且对细胞的基础呼吸无明显影响。同时,法舒地尔减弱了ROCK1过表达对线粒体裂变所需p-Drp1蛋白的表达的影响,也降低了肌肉萎缩相关蛋白MuRF1和Atrogin1蛋白的高表达。法舒地尔作为目前已在临床应用的药物,给未来应用于临床治疗肌肉萎缩提供了新的可能,但仍需要在动物实验中进一步证实。
志谢
感谢美国贝勒医学院胡兆永教授对本实验的帮助。
| [1] | LEVEY A S, CORESH J. Chronic kidney disease[J]. Lancet, 2012, 379: 165–180. DOI: 10.1016/S0140-6736(11)60178-5 |
| [2] | TAMAKI M, MIYASHITA K, WAKINO S, MITSUISHI M, HAYASHI K, ITOH H. Chronic kidney disease reduces muscle mitochondria and exercise endurance and its exacerbation by dietary protein through inactivation of pyruvate dehydrogenase[J]. Kidney Int, 2013, 85: 1330–1339. |
| [3] | JHA V, GARCIA-GARCIA G, ISEKI K, LI Z, NAICKER S, PLATTNER B, et al. Chronic kidney disease: global dimension and perspectives[J]. Lancet, 2013, 382: 260–272. DOI: 10.1016/S0140-6736(13)60687-X |
| [4] | ELGASS K, PAKAY J, RYAN M T, PALMER C S. Recent advances into the understanding of mitochondrial fission[J]. Biochim Biophys Acta, 2013, 1833: 150–161. DOI: 10.1016/j.bbamcr.2012.05.002 |
| [5] | DECLÈVES A E, SHARMA K. Novel targets of antifibrotic and anti-inflammatory treatment in CKD[J]. Nat Rev Nephrol, 2014, 10: 257–267. |
| [6] | HAGMANN H, BRINKKOETTER P T. ROS and oxidative stress in CKD patients: is it the mitochondria that keeps CKD patients in bed?[J]. Nephrol Dial Transplant, 2015, 30: 867–868. DOI: 10.1093/ndt/gfv052 |
| [7] | GRANATA S, DALLA GASSA A, TOMEI P, LUPO A, ZAZA G. Mitochondria: a new therapeutic target in chronic kidney disease [J/OL]. Nutr Metab (Lond), 2015, 12: 49. doi: 10.1186/s12986-015-0044-z.eCollection2015. |
| [8] | GAO H, HOU F, DONG R, WANG Z, ZHAO C, TANG W, et al. Rho-Kinase inhibitor fasudil suppresses high glucose-induced H9c2 cell apoptosis through activation of autophagy[J]. Cardiovasc Ther, 2016, 34: 352–359. DOI: 10.1111/cdr.2016.34.issue-5 |
| [9] | PENG H, CAO J, YU R, DANESH F, WANG Y, MITCH W E, et al. CKD stimulates muscle protein loss via Rho-associated protein kinase 1 activation[J/OL]. J Am Soc Nephrol, 2016, 27: 509-519. doi: 10.1681/ASN.2014121208. |
| [10] | WANG W, WANG Y, LONG J, WANG J, HAUDEK S B, OVERBEEK P, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells[J]. Cell Metab, 2012, 15: 186–200. DOI: 10.1016/j.cmet.2012.01.009 |
| [11] | FOLETTA V C, WHITE L J, LARSEN A E, LÉGER B, RUSSELL A P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy[J]. Pflugers Arch, 2011, 461: 325–335. DOI: 10.1007/s00424-010-0919-9 |
| [12] | WORKENEH B T, MITCH W E. Review of muscle wasting associated with chronic kidney disease[J]. Am J Clin Nutr, 2010, 91: 1128S–1132S. DOI: 10.3945/ajcn.2010.28608B |
| [13] | YOKOI H, YANAGITA M. Decrease of muscle volume in chronic kidney disease: the role of mitochondria in skeletal muscle[J]. Kidney Int, 2014, 85: 1258–1260. DOI: 10.1038/ki.2013.539 |
| [14] | WANG X H, MITCH W E. Mechanisms of muscle wasting in chronic kidney disease[J]. Nat Rev Nephrol, 2014, 10: 504–516. DOI: 10.1038/nrneph.2014.112 |
| [15] | CARRERO J J, STENVINKEL P, CUPPARI L, IKIZLER T A, KALANTAR-ZADEH K, KAYSEN G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease-a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM)[J]. J Ren Nutr, 2013, 23: 77–90. DOI: 10.1053/j.jrn.2013.01.001 |
| [16] | IKIZLER T A, CANO N J, FRANCH H, FOUQUE D, HIMMELFARB J, KALANTAR-ZADEH K, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism[J]. Kidney Int, 2013, 84: 1096–1107. DOI: 10.1038/ki.2013.147 |
| [17] | ROMANELLO V, GUADAGNIN E, GOMES L, RODER I, SANDRI C, PETERSEN Y, et al. Mitochondrial fission and remodelling contributes to muscle atrophy[J]. EMBO J, 2010, 29: 1774–1185. DOI: 10.1038/emboj.2010.60 |
 2017, Vol. 38
2017, Vol. 38


