2. 中山大学附属第三医院肝脏外科, 广州 510630
2. Department of Liver Surgery, Third Affiliated Hospital, Sun Yat-sen University, Guangzhou 510630, Guangdong, China
外科脑损伤(surgical brain injury, SBI)是指在神经外科手术在切除病灶过程中不可避免地对周围正常脑组织造成的破坏,关键部位的损伤会引起听觉、视觉、语言及运动功能障碍[1];而手术引起的原发和继发性炎症反应会进一步破坏血脑屏障,最终导致颅内高压, 甚至死亡[2],因而抗炎治疗是SBI发生后的关键措施。但目前使用的皮质激素类抗炎制剂及非甾类抗炎药物均可引发不确定的不良反应和毒性反应[3],因而需要寻找更有效的炎症控制方案。
5-脂氧合酶(5-lipoxygenase, 5-LOX)是催化花生四烯酸(arachidonic acid, AA)产生白三烯(leukotriene, LT)炎症介质的关键酶,LT可在损伤的脑组织中诱导强烈的炎症反应[4]。核转录因子(NF)-κB是脑损伤过程中重要的调控因子,当机体发生应激反应时,NF-κB被激活并转移至核内促进多种炎症因子的转录,从而调控炎症反应[5]。近年研究表明,过氧化反应可通过加重细胞膜损伤、影响ATP酶活性、抑制钙流出、参与介导炎症级联反应等影响脑损伤后的细胞功能,进而加重脑损伤[6~9]。炎症和过氧化反应在创伤性和缺血性脑损伤中的作用已有较多研究[10~12],且目前对5-LOX的报道多关注其上述作为炎症反应限速酶的酶性作用,而对5-LOX的非酶作用及其与过氧化反应的关系尚未见报道。此外,5-LOX在SBI脑组织中的时空表达规律也缺乏相关资料;而这些对阐明5-LOX的作用机制并寻找干预其表达的最佳时间点以有效地减缓脑损伤具有重要意义。本实验采用右额叶切除术建立大鼠SBI模型,并在SBI术后不同时间点收集各组大鼠脑组织标本,对损伤侧和健侧脑组织进行比较研究,试图阐明5-LOX在SBI脑组织中的表达规律及其可能的作用机制。
1 材料和方法 1.1 实验对象与分组处理将72只体质量为250~300 g [南方医科大学实验动物中心提供,使用许可证号:SYXK(粤)2012-0117]的SPF级健康成年雄性SD大鼠按随机数表法分为假手术(Sham)组及SBI术后1 d (SBI-1d)组、3 d (SBI-3d)组、7 d (SBI-7d)组,每组18只。动物饲养在暨南大学实验动物中心,饮食和日照等符合国家实验动物饲养标准。对SBI-1d、SBI-3d和SBI-7d组大鼠采用右额叶切除法建立SBI模型,具体操作方法参照文献[13];对Sham组大鼠只移除相应部位的颅骨且不伤及硬脑膜。
1.2 脑组织含水量测定深麻取脑,在冰上将整个大脑分为损伤同侧与损伤对侧两部分,用滤纸吸干脑表面水分,用电子分析天平称湿质量;然后置于105 ℃恒温干燥箱内干燥72 h后称其干质量;按公式计算脑含水量,脑含水量(%)=(湿质量-干质量)/湿质量×100%。
1.3 神经行为学评分采用改良的Garcia评分和杠杆平衡评分[14~15]分别评价各组大鼠的感觉运动能力和平衡能力。Garcia评分包括7项测试,每项测试的分值是0~3分,满分21分;在杠杆平衡测试中,杠杆置于2个平台中间,将大鼠放在杠杆的中间位置,观察大鼠在杠杆上停留时间以及能够走向任意平台的时间,分值为0~5分;具体评分标准操作参照文献[14]。
1.4 脑组织中超氧化物歧化酶(superoxidedismutase, SOD)活性和丙二醛(malondioldehyde, MDA)含量的测定对各组大鼠分别深麻、断头取脑,在损伤区周围取脑组织0.5 g,加生理盐水稀释10倍,用玻璃匀浆器在冰上研磨,制成脑组织匀浆,616×g 4 ℃离心15 min,取适量上清分别进行蛋白定量、MDA含量和SOD活性测定。蛋白定量、MDA和SOD测定试剂盒均由南京建成生物工程研究所提供,具体操作按相应说明书进行。
1.5 脑组织中5-LOX的免疫荧光染色用4%多聚甲醛溶液灌注,取脑固定后冰冻切片,层厚5 μm。切片用PBS洗3次后,用含0.3% Triton X-100、5%驴血清的PBS室温封闭30 min;4 ℃过夜标记一抗:5-LOX(Cayman公司,货号:160402;1:500比例稀释)、胶质纤维酸性蛋白(glial fibrillary acidic protein,GFAP;Abcam公司,货号:ab10062;1:500比例稀释)、神经元特异性核蛋白(NeuN;Abcam公司,货号:ab104224;1:400比例稀释)、离子钙接头蛋白分子1 (Iba-1;Abcam公司,货号:ab15690;1:400比例稀释);次日复温后用PBS洗3次,室温避光标记相应二抗90 min,PBS洗3次;DAPI染核5 min,50%甘油封片。采用荧光显微镜(Leica DM600) 观察并拍照。
1.6 蛋白质印迹法检测5-LOX和NF-κB的蛋白表达冰上取损伤周围脑组织和对侧相应部位的脑组织各0.5 g,裂解细胞提取蛋白,聚丙烯酰胺凝胶电泳分离目的蛋白,PVDF膜转膜后加入适量封闭液封闭,4 ℃摇床过夜标记5-LOX多克隆抗体(Cayman公司,货号:160402;1:1 000比例稀释)和NF-κB多克隆抗体(Cell Signaling Technology公司,货号:6956S;1:1 000比例稀释),以GAPDH(Cell Signaling Technology公司,货号:5174;1:1 000比例稀释)作为内参蛋白,次日洗膜后室温标记相应二抗1 h,曝光保存。用ImageJ软件分析条带灰度值。
1.7 统计学处理应用SPSS 19.0软件进行数据分析。所有计量资料以x±s表示,多组间比较采用单因素方差分析(one-way ANOVA),进一步两组间比较采用SNK法。检验水准(α)为0.05。
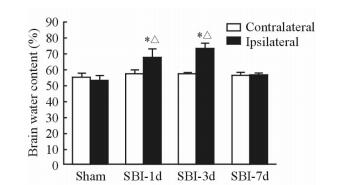
2 结果 2.1 SBI后脑组织含水量的改变SBI大鼠损伤侧脑组织含水量呈先增加后恢复的趋势,SBI-3d组脑组织含水量最高。与Sham组相比,SBI-1d和SBI-3d组大鼠损伤侧脑组织含水量增加(P<0.05);与SBI-7d组大鼠脑组织含水量相比,SBI-1d和SBI-3d组大鼠损伤侧脑组织含水量差异有统计学意义(P<0.05)。见图 1。
|
图 1 各组大鼠脑组织含水量 Fig 1 Brain water content of rats in each group SBI-1d: 1 d post surgical brain injury (SBI); SBI-3d: 3 d post SBI; SBI-7d: 7 d post SBI. *P < 0.05 vs Sham group; △P < 0.05 vs SBI-7d group. n=6, x±s |
2.2 SBI后神经功能的改变
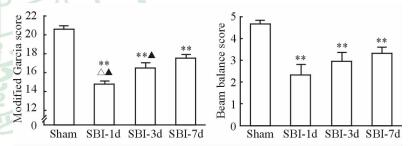
SBI-1d、SBI-3d和SBI-7d组大鼠的Garcia评分低于Sham组(P<0.01),SBI-1d、SBI-3d组均低于SBI-7d组(P<0.05),且SBI-1d组与SBI-3d组差异有统计学意义(P<0.05,图 2)。在杠杆平衡评分方面,与Sham组相比,SBI-1d、SBI-3d和SBI-7d组大鼠均出现明显的神经功能障碍(P<0.01),但SBI-1d、SBI-3d和SBI-7d组之间差异无统计学意义(图 2)。
|
图 2 各组大鼠神经功能学评分 Fig 2 Neurobehavior scores of rats in each group SBI-1d: 1 d post surgical brain injury (SBI); SBI-3d: 3 d post SBI; SBI-7d: 7 d post SBI. **P < 0.01 vs Sham group; △P < 0.05 vs SBI-3d group; ▲P < 0.05 vs SBI-7d group. n=6, x±s |
2.3 SBI后5-LOX免疫荧光染色结果
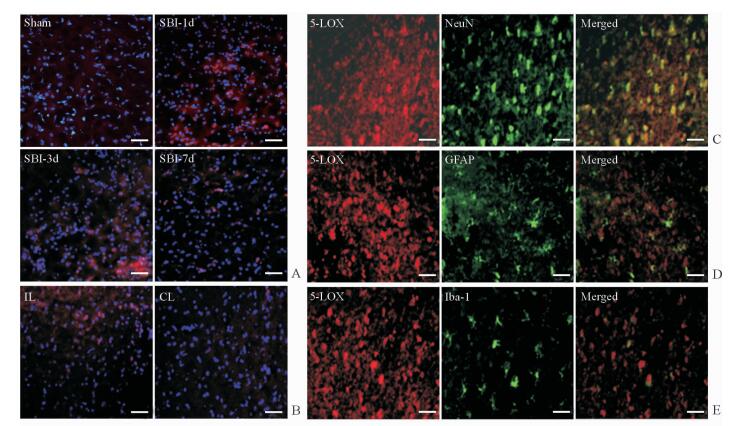
由图 3A可见,5-LOX在Sham组几乎不表达,在SBI-1d、SBI-3d组表达增加,SBI-7d组表达减少。5-LOX在损伤同侧损伤区周围的脑组织中表达较多,而在对侧正常脑组织中几乎无表达(图 3B)。由细胞分布和定位图可见,5-LOX在脑组织中的表达以神经元为主,在星形胶质细胞和小胶质细胞也有阳性着色(图 3C~3E)。
|
图 3 SBI后大鼠脑组织中5-LOX的时间表达和细胞定位 Fig 3 Temporal expression and cellular localization of 5-LOX in rat brain tissues after surgical brain injury (SBI) A: The temporal expression of 5-lipoxygenase (5-LOX) in the ipsilateral (IL) brain tissues of rats in the Sham, SBI-1d, SBI-3d and SBI-7d groups; B: 5-LOX expression in the IL and contralateral (CL) brain tissues of rats at 1 day after SBI; C: Co-localization of 5-LOX with neuronal nuclear antigen (NeuN), a biomarker for neurons, at 1 day after SBI; D: Co-localization of 5-LOX with glial fibrillary acidic protein (GFAP), an astrocytes marker, at 1 day after SBI; E: Co-localization of 5-LOX with ionized calcium binding adaptor molecule-1 (Iba-1), a microglia biomarker, at 1 day after SBI. SBI-1d: 1 d post SBI; SBI-3d: 3 d post SBI; SBI-7d: 7 d post SBI. Red color indicates the expression of 5-LOX, blue indicates nucleus, and green indicates NeuN in Fig 3C, GFAP in Fig 3D, and Iba-1 in Fig 3E. Scale bar=50 μm |
2.4 SBI后5-LOX和NF-κB表达的变化
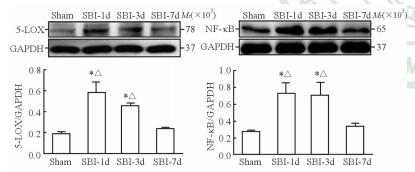
与Sham组相比,SBI-1d和SBI-3d组大鼠损伤区周围脑组织中5-LOX与NF-κB蛋白表达均增加(P<0.05);与SBI-1d和SBI-3d组相比,SBI-7d组的5-LOX和NF-κB表达均降低,差异有统计学意义(P<0.05)。见图 4。
|
图 4 各组大鼠损伤区周围脑组织中5-LOX和NF-κB的蛋白表达 Fig 4 Protein expression of 5-LOX and NF-κB of brain tissue around lesion areas in each group 5-LOX: 5-Lipoxygenase; NF-κB: Nuclear factor κB; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. SBI-1d: 1 d post surgical brain injury (SBI); SBI-3d: 3 d post SBI; SBI-7d: 7 d post SBI. *P < 0.05 vs Sham group; △P < 0.05 vs SBI-7d group. n=4, x±s |
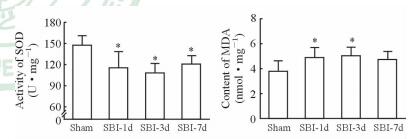
2.5 SBI后脑组织SOD活性及MDA含量的改变
与Sham组相比,SBI-1d、SBI-3d、SBI-7d组大鼠损伤区周围脑组织中SOD活性降低(P<0.05);SBI-1d和SBI-3d组MDA含量明显升高(P<0.05),但SBI-7d组MDA含量与Sham组差异无统计学意义(P>0.05)。见图 5。
|
图 5 各组大鼠脑组织SOD活性和MDA含量 Fig 5 Activity of SOD and content of MDA in each group SOD: Superoxide dismutase; MDA: Malondialdehyde. SBI-1d: 1 d post surgical brain injury (SBI); SBI-3d: 3 d post SBI; SBI-7d: 7 d post SBI. *P < 0.05 vs Sham group. n=6, x±s |
3 讨论
在脑外科手术过程中,刀片切割的创伤、电凝器的灼伤、牵引器的拉伤等导致脑组织不可避免地损伤,而继发性出血、炎症和水肿等进一步加重脑损伤[16]。当手术的直接损伤无法避免时,有效止血、抑制炎症反应和减轻氧化应激等可以在一定程度上缓解症状、缩短病程和促进术后神经功能的恢复。虽然目前抗炎和利尿等对症治疗可以改善外科手术后的炎症和水肿[3, 17],但疗效不确定同时还可能引发严重的不良反应[18],因而进一步探讨SBI的发病机制以寻找更有效的治疗靶点仍是值得研究的话题。5-LOX是经典炎症通路AA—5-LOX—LT中的限速酶,其活性决定了炎症趋化因子的水平,因而在炎症反应中扮演着重要角色[19~21],由于关于这方面的报道已经较多,因此本实验重点研究5-LOX的时空表达规律,并探讨其酶性致炎作用之外的其他可能机制。
本实验采用右额叶切除术建立大鼠SBI模型。脑组织含水量测定可以客观地评价术后脑水肿的程度,本实验结果显示大鼠在SBI术后1 d、3 d发生严重的脑水肿,术后7 d有所改善。神经功能评分是评价脑损伤的最重要指标之一,其中Garcia评分是相对灵敏的评分标准[22],主要评价大鼠术后的感觉运动能力,而杠杆平衡评分可以判断大鼠术后的平衡能力。本实验发现SBI后,大鼠Garcia评分先下降然后逐渐恢复,可能与神经组织存在部分自我修复能力有关[23]。上述结果表明该模型较好地模拟了临床SBI的病理过程。
SBI后5-LOX表达在时间和空间上有一定规律:在时间上,5-LOX表达在损伤后第1天增加,第3天有所下降,到第7天逐渐恢复;在空间上,5-LOX主要分布在脑损伤区域的周围组织,在对侧和远离损伤区的健康脑组织中几乎不表达,且5-LOX主要定位于细胞胞质,在神经元表达较多,星形胶质细胞和小胶质细胞中也有阳性表达。术后1~3 d属于炎症反应急性期[24~25],各项炎症指标发生明显变化。术后7 d是由急性炎症期过渡到慢性炎症期和代偿恢复期的一个临界点,此时引起5-LOX表达增加的因素,如Ca2+和其他细胞毒性产物等被逐步改善的血液循环清除,因而5-LOX表达也逐渐减少;机体的炎症反应也激活了机体内源性脂质抗炎和促炎症消退的介质——脂氧素的生成,5-LOX的表达减少也可能与此“刹车信号”有关[26]。此外,随着白细胞介导的急性炎症消退,其伴随的氧化应激也得到缓解;同时,体内自由基清除(抗过氧化)机制被启动,所以相关指标逐渐恢复[27]。
本研究免疫荧光染色结果显示5-LOX在损伤区周围的神经元胞质中大量表达,在星形胶质细胞和小胶质细胞中也有表达。表达增多的5-LOX在各种损伤因子的作用下被激活,通过经典的LT途径引起炎症反应,而在胶质细胞中表达增多的5-LOX则可能加重神经元损伤[28]。SOD是体内重要的抗氧化酶,其活性可反映机体清除氧自由基的能力;而MDA是脂质过氧化反应的产物,其含量高低间接反映细胞受自由基攻击的严重程度[23]。本研究结果显示,大鼠SBI后脑组织中MDA含量较Sham组增加,而SOD活性明显下降;这在时间点上与5-LOX表达呈一致性。据报道,5-LOX通路的激活在生成大量LT的同时也可产生羟自由基(·OH),而脑组织中的膜结构含有丰富的多不饱和脂肪酸,极易与·OH反应启动脂质过氧化反应;采用5-LOX抑制剂zileuton可以通过抑制AA代谢的5-LOX通路减少自由基的生成,从而抑制脑组织脂质过氧化反应[29]。所以,5-LOX除了通过上述炎症通路,也可能通过加重氧化应激参与脑损伤的病理过程。
5-LOX在中枢神经系统的作用不仅与其上述的酶功能有关,也可能与其非酶功能相关。研究表明创伤性脑损伤后脑组织NF-κB的转录活性增强[30];而5-LOX在NF-κB-IκB复合体形成过程中可能发挥作用,且其活化能使NF-κB易位到核内,进而影响转录[31];5-LOX的激活可促进NF-κB的表达和活化,活化的NF-κB进一步诱导NF-κB依赖性细胞因子、趋化因子、黏附因子以及神经肽的表达,引起级联炎症反应[32];另外5-LOX的下游产物白三烯B4 (leukotriene B4, LTB4) 能够促进NF-κB与DNA结合,形成恶性循环而扩大炎症反应[4, 10]。本研究结果显示,在SBI后损伤侧周围脑组织中5-LOX表达增加,3 d后逐渐下降;而NF-κB表达趋势与5-LOX一致,提示5-LOX可能通过与NF-κB相互作用加重炎症反应,进而加重脑损伤,但仍需进一步研究明确这一论点。
综上所述,5-LOX在SBI后表达增加,除了作为炎症通路的限速酶直接发挥作用外,5-LOX加重脑损伤可能与脑损伤后NF-κB表达增加和氧化应激加重有关。可见,5-LOX在SBI病理过程中发挥重要作用,对其进行有效干预可能有良好的应用前景。但本研究并未使用特异性阻断剂抑制5-LOX表达后再检测NF-κB、SOD和MDA等指标的变化,所以尚不能确定5-LOX与NF-κB和氧化应激之间是否直接相关,这需要我们进一步研究。
| [1] | BENGGON M, CHEN H, APPLEGATE R, MARTIN R, ZHANG J H. Effect of dexmedetomidine on brain edema and neurological outcomes in surgical injury in rats[J]. Anesth Analg, 2012, 115: 154–159. DOI: 10.1213/ANE.0b013e31824e2b86 |
| [2] | HAMARD L, RATEL D, SELEK L, BERGER F, VAN DER SANDEN B, WION D. The brain tissue response to surgical injury and its possible contribution to glioma recurrence[J]. J Neurooncol, 2016, 128: 1–8. DOI: 10.1007/s11060-016-2096-y |
| [3] | LI Q, XU M, ZHOU J X. Correlation of measured and calculated serum osmolality during mannitol or hypertonic saline infusion in patients after craniotomy: a study protocol and statistical analysis plan for a randomised controlled trial[J/OL]. BMJ Open, 2014, 4: e004921.doi: 10.1136/bmjopen-2014-004921. |
| [4] | 余涓, 吴艳云, 翁绳美. 5-LOX通路活化对大鼠脑缺血再灌注损伤致血脑屏障破坏的影响[J]. 神经解剖学杂志, 2009, 25: 177–183. |
| [5] | 杨帆, 王永青, 彭余江, 邵波, 朱良才, 段红宇, 等. NF-κB在颅脑损伤后继发氧化应激及细胞凋亡之间的关系研究[J]. 浙江创伤外科, 2014, 19: 899–902. DOI: 10.3969/j.issn.1009-7147.2014.06.008 |
| [6] | HYONG A, JADHAV V, LEE S, TONG W, ROWE J, ZHANG J H. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents[J]. Brain Res, 2008, 1215: 218–224. DOI: 10.1016/j.brainres.2008.04.025 |
| [7] | MUSTAFA A G, SINGH I N, WANG J, CARRICO K M, HALL E D. Mitochondrial protection after traumatic brain injury by scavenging lipid peroxyl radicals[J]. J Neurochem, 2010, 114: 271–280. |
| [8] | HALL E D, VAISHNAV R A, MUSTAFA A G. Antioxidant therapies for traumatic brain injury[J]. Neurotherapeutics, 2010, 7: 51–61. DOI: 10.1016/j.nurt.2009.10.021 |
| [9] | 荆俊杰, 陈锦华, 魏梁锋, 赵清爽, 应建彬, 王守森, 等. 大鼠二次脑损伤后脑组织SOD及MDA的变化研究[J]. 现代生物医学进展, 2015, 15: 812–814. |
| [10] | JATANA M, CIRI S, ANSARI M A, ELANGO C, SINGQH I, KHAN M. Inhibition of NF-κB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia[J]. J Neuroinflammation, 2006, 3: 13. DOI: 10.1186/1742-2094-3-13 |
| [11] | 阳又莲, 张新, 罗扬, 刘宿. 姜黄素改善大鼠创伤性脑损伤炎性反应及预后[J]. 现代医药卫生, 2015, 20: 3056–3058. DOI: 10.3969/j.issn.1009-5519.2015.20.004 |
| [12] | 孔祥溢, 关健, 王任直. 氧化应激在急性脑缺血病程中的分子生物学作用[J]. 中国医学科学院学报, 2016, 38: 222–227. DOI: 10.3881/j.issn.1000-503X.2016.02.017 |
| [13] | JADHAV V, SOLAROGLU I, OBENAUS A, ZHANG J H. Neuroprotection against surgically induced brain injury[J]. Surg Neurol, 2007, 67: 15–20. DOI: 10.1016/j.surneu.2006.07.014 |
| [14] | McBRIDE D W, WANG Y, SHERCHAN P, TANG J, ZHANG J H. Correlation between subacute sensorimotor deficits and brain water content after surgical brain injury in rats[J]. BehavBrainRes, 2015, 290: 161–171. |
| [15] | GARCIA J H, WAGNER S, LIU K F, HU X J. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats[J]. Stroke, 1995, 26: 627–634. DOI: 10.1161/01.STR.26.4.627 |
| [16] | 郝崴, 张莹莹, 徐荣天. 氨基胍治疗大鼠手术性脑损伤致脑水肿模型的磁共振灌注研究[J]. 临床放射学杂志, 2011, 30: 414–416. |
| [17] | HUANG L, SHERCHAN P, WANG Y, REIS C, APPLEQATE R L, TANG J. Phosphoinositide 3-kinase gamma contributes to neuroinflammation in a rat model of surgical brain injury[J]. J Neurosci, 2015, 35: 10390–10401. DOI: 10.1523/JNEUROSCI.0546-15.2015 |
| [18] | 张声, 蔡文训, 罗华, 李卓. 颅脑损伤的非手术脑保护措施进展[J]. 当代医学, 2012, 18: 26–28. DOI: 10.3969/j.issn.1009-4393.2012.14.015 |
| [19] | ZHANG X Y, CHEN L, YANG Y, XU D M, ZHANG S R, LI C T, et al. Regulation of rotenone-induced microglial activation by 5-lipoxygenase and cysteinyl leukotriene receptor 1[J]. Brain Res, 2014, 1572: 59–71. DOI: 10.1016/j.brainres.2014.05.026 |
| [20] | YOKOMIZO T, UOZUMI N, TAKAHASHI T, KUME K, IZUMI T, SHIMIZU T. Leukotriene A4 hydrolase and leukotriene B4 metabolism[J]. J Lipid Mediat Cell Signal, 1995, 12: 321–332. DOI: 10.1016/0929-7855(95)00041-N |
| [21] | TU X K, YANG W Z, SHI S S, CHEN C M, WANG C H. 5-lipoxygenase inhibitor zileuton attenuates ischemic brain damage: involvement of matrix metalloproteinase 9[J]. Neurol Res, 2009, 31: 848–852. DOI: 10.1179/174313209X403913 |
| [22] | ABE T, KUNZ A, SHIMAMURA M, ZHOU P, ANRATHER J, IADECOLA C. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic[J]. J Cereb Blood Flow Metab, 2009, 29: 66–72. DOI: 10.1038/jcbfm.2008.88 |
| [23] | 夏磊, 陈泉, 程冠亮, 姜正林, 高志伟. 人参总皂苷对大鼠创伤性脑水肿的影响[J]. 中国中西医结合杂志, 2012, 32: 1671–1674. |
| [24] | AYER R E, JAFARIAN N, CHEN W, APPLEGATE R L 2nd, COLOHAN A R, ZHANG J H. Preoperative mucosal tolerance to brain antigens and a neuroprotective immune response following surgical brain injury[J]. J Neurosurg, 2012, 116: 246–253. DOI: 10.3171/2011.8.JNS11883 |
| [25] | SERHAN C N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways[J]. Annu Rev Immunol, 2007, 25: 101–137. DOI: 10.1146/annurev.immunol.25.022106.141647 |
| [26] | LEHMANN C, HOMANN J, BALL A K, BLÖCHER R, KLEINSCHMIDT T K, BASAVARAJAPPA D. Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein[J]. FASEB J, 2015, 29: 5025–5043. |
| [27] | 韩宁. 大鼠脑出血模型中神经细胞凋亡与自由基水平的相关性研究[D]. 上海: 第二军医大学, 2007. |
| [28] | ZHANG L, ZHANG W P, HU H, WANG M L, SHRNH W W, YAO H T, et al. Expression patterns of 5-lipoxygenase in human brain with traumatic injury and astrocytoma[J]. Neuropathology, 2006, 26: 99–106. DOI: 10.1111/neu.2006.26.issue-2 |
| [29] | 许琳, 余涓, 刘颖, 陈崇宏. 5-脂氧合酶抑制剂zileuton对大鼠局灶性脑缺血/再灌注损伤的保护作用[J]. 中国药理学通报, 2006, 22: 853–855. DOI: 10.3321/j.issn:1001-1978.2006.07.021 |
| [30] | 刘盛明, 苑玉清, 侯敏, 吴红. 创伤性脑损伤脑组织NF-κB表达变化规律及PDTC对其影响[J]. 四川医学, 2013, 34: 167–169. DOI: 10.3969/j.issn.1004-0501.2013.02.009 |
| [31] | LEPLEY R A, FITZPATRICK F A. 5-Lipoxygenase compartmentalization in granulocytic cells is modulated by an internal bipartite nuclear localizing sequence and nuclear factor κB complex formation[J]. Arch Biochem Biophys, 1998, 356: 71–76. DOI: 10.1006/abbi.1998.0744 |
| [32] | 王晓晨, 吉爱国. NF-κB信号通路与炎性反应[J]. 生理科学进展, 2014, 45: 68–71. |
 2017, Vol. 38
2017, Vol. 38


