2. 南通大学医学院解剖教研室, 南通 226001;
3. 上海交通大学附属第六人民医院妇产科, 上海 200233
2. Department of Anatomy, School of Medicine, Nantong University, Nantong 226001, Jiangsu, China;
3. Department of Obstetrics and Gynecology, Sixth People's Hospital of Shanghai, Shanghai Jiaotong University, Shanghai 200233, China
卵巢癌是妇科目前第二常见、病死率最高的肿瘤,据统计全世界每年新增238 700例卵巢癌患者[1]。大部分卵巢癌患者在就诊时已处于晚期[国际妇产科联盟大会(FIGO)Ⅲ~Ⅳ期],且多数发生腹腔内转移[2]。发生转移是卵巢癌患者的主要病死原因,但不幸的是其转移机制仍不清楚。
同源盒基因是指含有180 bp的高度保守的同源盒DNA序列的基因,能够通过同源域蛋白抑制或者激活靶基因的表达,在调节细胞分化、生长和发育过程中发挥重要作用[3]。LIM同源盒基因8 (LIM homeobox gene-8,Lhx8) 含有2个重复串联的富含半胱氨酸的双锌指结构(LIM结构域)和1个DNA结合的同源结构域,参与了多种组织的分化过程[3]。在早期卵子分裂时,Lhx8在卵母细胞分化和维持过程中扮演着重要角色[4];并且对裸鼠模型研究发现,Lhx8是牙胚间充质细胞生存的重要因素[5]。Lhx8对基底前脑胆碱能神经元的形成和功能维持具有重要作用[6]。目前研究显示Lhx家族的一些成员与肿瘤发生有关[3-4],然而尚无研究报道Lhx8在卵巢癌细胞生物行为过程中的作用。本研究通过在人卵巢癌细胞株SKOV3中过表达或干扰Lhx8,探讨Lhx8对卵巢癌细胞增殖、迁移和侵袭的影响。
1 材料和方法 1.1 细胞培养人卵巢癌细胞株SKOV3购自广州行知生物科技有限公司。细胞用含10%胎牛血清(FBS,Gibco)、100 U/mL青霉素和100 μg/mL链霉素的RPMI 1640培养液(Gibco)培养于37 ℃、5% CO2培养箱中。细胞融合率达90%时进行传代,第2~3代细胞用于实验。
1.2 Lhx8过表达细胞模型构建使用GV287慢病毒表达系统(上海吉玛制药技术有限公司)构建Lhx8过表达慢病毒(LV-Lhx8) 和阴性对照慢病毒(LV-NC)。将SKOV3细胞种植于6孔板中,细胞密度为1×105/孔,培养24 h后分别加入LV-Lhx8、LV-NC转染细胞,野生(WT)组细胞给予等量不含病毒的转染试剂处理,48 h后收集细胞。通过免疫荧光和蛋白质印迹法观察过表达效果。
1.3 Lhx8干扰细胞模型构建合成3种目的基因的siRNA序列。Lhx8-siRNA-1:sense 5′-GAU GCU CAU UCA CCA ACA ATT-3′, anti-sense 5′-UUG UUG GUG AAU GAG CAU CTT-3′;Lhx8-siRNA-2:sense 5′-CCA CCC AUG UUA GAA GAA ATT-3′,anti-sense 5′-UUU CUU CUA ACA UGG GUG GTT-3′;Lhx8-siRNA-3:sense 5′-GCA UGC UGG AUA AUU UAA ATT-3′,anti-sense 5′-UUU AAA UUA UCC AGC AUG CTT-3′。同时合成标记羧基荧光素(carboxyfluorescein mixed isomers,FAM)的阴性对照序列(FAM-siRNA)。培养细胞,使用Lipofectamine 2000将3种Lhx8-siRNA和FAM-siRNA分别转入SKOV3细胞,WT组细胞仅给予不加任何干扰片段的转染试剂处理。于培养6 h和24 h后分别采用qPCR和蛋白质印迹法验证干扰效果。
1.4 蛋白质印迹法按照胞核-胞质蛋白制备试剂盒(北京普利莱基因技术有限公司)说明书操作说明提取细胞的胞质胞核蛋白。利用BCA蛋白浓度测定试剂盒(上海碧云天生物技术研究所)蛋白定量后,进行SDS-PAGE、转膜(NC膜);用1%的小牛血清白蛋白(bovine serum albumin,BSA)室温封闭NC膜1 h;然后4 ℃过夜标记一抗:Lhx8(稀释比例1:500,Abcam)、基质金属蛋白酶(matrix metalloproteinase,MMP)-2 (稀释比例1:1 000,Abcam)、MMP-9 (稀释比例1:1 000,Abcam),以β-actin(稀释比例1:15 000,Sigma)作为内参蛋白;次日标记相应二抗。采用自动电泳凝胶成像分析仪(Bio-Rad)采集结果并计算灰度值。
1.5 RNA的提取和qPCR反应使用TRIzol试剂(TaKaRa)提取细胞总RNA。采用反转录试剂盒(TaKaRa)反转录获得cDNA,以cDNA为模板、采用Corbett Rotor-Gene 6000 qPCR仪进行qPCR反应。引物序列:Lhx8 sense 5′-GTA TCA CTT GGC TTG CTT-3′、anti-sense:5′-ATT ACC GTT CTC CAC TTC-3′,内参基因GAPDH sense 5′-ACC ACA GTC CAT GCC ATC AC-3′、anti-sense 5′-TCC ACC ACC CTG TTG CTG TA-3′。反应条件:95 ℃ 5 min;95 ℃ 15 s、56 ℃ 30 s、72 ℃ 40 s,40个循环;72~95 ℃收集荧光信号行熔解曲线分析。计算Ct值,采用Pfaffl法[7]分析结果,将Lhx8和GAPDH比值作为Lhx8基因mRNA的相对表达量。
1.6 EDU实验将SKOV-3细胞种植于24孔板,调整细胞密度为2×104/孔。培养24 h后,加入50 μmol/L EDU孵育2 h,然后用Apollo-567荧光染料染色30 min,Hoechst对细胞核进行复染。使用Olympus激光共聚焦显微镜观察细胞荧光表达情况。
1.7 Transwell实验将SKOV-3细胞悬浮于含0.2% FBS的RPMI 1640培养液中,取200 μL(含2×104细胞)加入24孔Transwell上室;下室加入500 μL含10% FBS的RPMI 1640培养液。在37 ℃、5% CO2条件下培养24 h后,用棉签去除上室底部未侵袭细胞,膜滤器固定,结晶紫染色,用Olympus倒置显微镜随机取4个视野观察、计数。
1.8 划痕实验细胞接种于24孔板中,生长融合到80%~90%,用枪头进行划痕、PBS清洗。分别在划痕后和培养18 h时进行拍照,以评估细胞迁移能力。
1.9 免疫荧光染色细胞培养24 h后,依次用2%甲醛固定、PBS(pH=7.4) 冲洗15 min、Triton X-100透化、PBS冲洗1 min、3% BSA封闭1 h,然后4 ℃过夜标记Lhx8兔单抗(稀释比例1:300,Abcam)。室温下标记Alexa Fluor® 594二抗(稀释比例1:1 000,Life Technologies) 1 h后,Hoechst染核20 min。利用激光共聚焦显微镜观察并采集图像。
1.10 细胞周期实验细胞于6孔板培养24 h后,用1 mmol/L胸苷(Sigma)处理细胞24 h使同步在G1/S交界期。然后用无血清培养液培养24 h,胰酶消化,预冷PBS清洗2次,乙醇4 ℃过夜固定,然后用PBS清洗2次。在室温下,用含0.2 mg/mL RNase A和20 μg/mL碘化丙啶(PI)的PBS孵育15 min。最后用流式细胞仪(BD)分析细胞周期。
1.11 统计学处理应用SPSS 16.0软件进行数据分析。数据以x±s表示,多样本均值的比较采用方差分析。检验水准(α)为0.05。
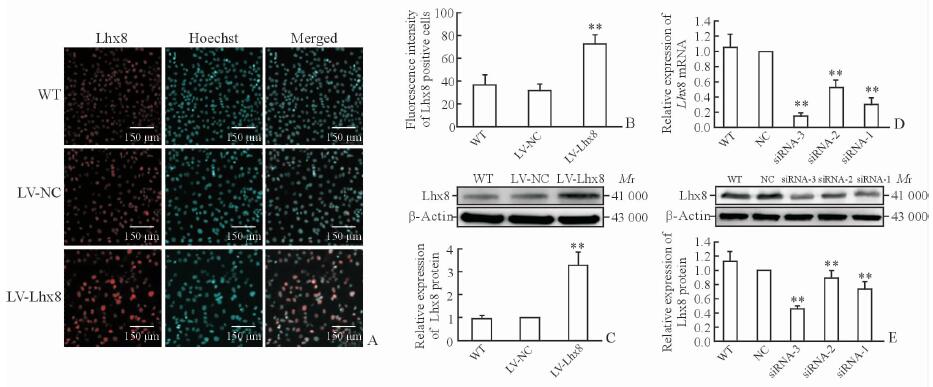
2 结果 2.1 过表达和干扰效果鉴定为明确Lhx8对卵巢癌细胞生物学行为的影响,我们用LV-Lhx8转染SKOV3细胞,同时设置LV-NC和WT型为对照组。转染48 h后通过免疫荧光染色检测,结果显示LV-Lhx8组Lhx8的表达多于LV-NC、WT组(P<0.01,图 1A、1B),同时蛋白质印迹法结果显示,LV-Lhx8组Lhx8蛋白表达较LV-NC和WT组相比上调(P<0.01,图 1C)。
|
图 1 Lhx8过表达和干扰后SKOV3细胞LIM同源盒基因8的表达情况 Fig 1 LIM homeobox gene-8 (Lhx8) expression in SKOV3 cells transfected with LV-Lhx8 or Lhx8-siRNA A, B: Immunofluorescence results of Lhx8; C: Western blotting results of Lhx8 expression in SKOV3 cells transfected with lentivirus-mediated Lhx8 overexpression (LV-Lhx8); D: The relative expression of Lhx8 mRNA normalibized by NC group; E: Western blotting results of Lhx8 expression in SKOV3 cells transfected with Lhx8-targeted small interfering RNA (Lhx8-siRNA). **P < 0.01 vs LV-NC and WT groups in Fig 1B and 1C, and vs NC and WT groups in Fig 1D and 1E. n=3, x±s |
此外,我们分别转染3种Lhx8-siRNA对SKOV3细胞进行干扰,干扰后Lhx8蛋白和mRNA的表达均低于NC和WT组,且以Lhx8-siRNA-3降低最为明显(P<0.01,图 1D、1E),因此将Lhx8-siRNA-3干扰的细胞模型用于后续实验。
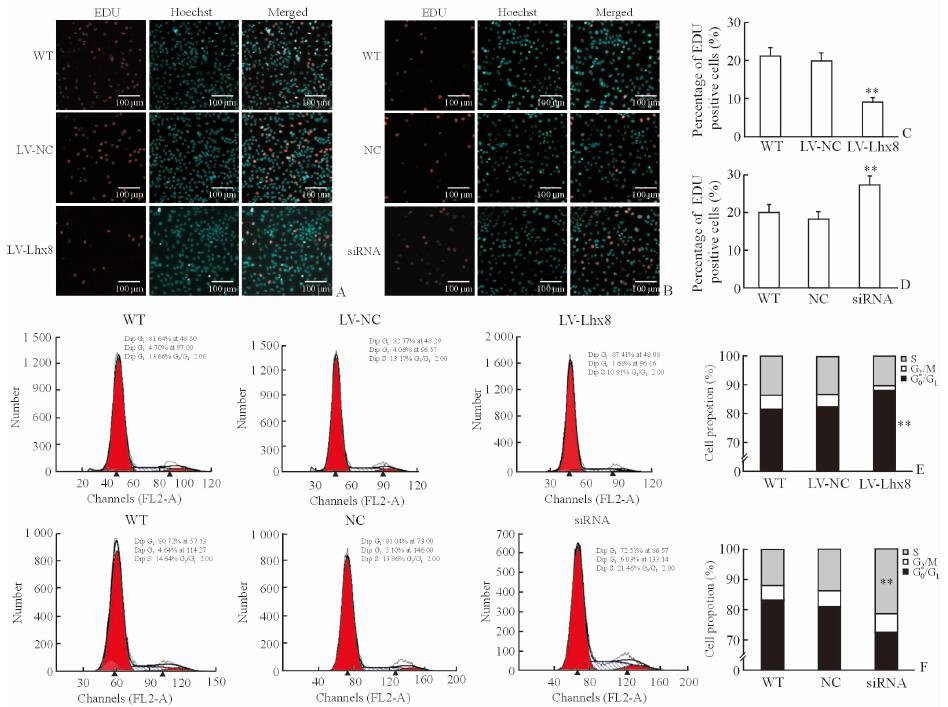
2.2 Lhx8对SKOV3细胞增殖的影响与LV-NC和WT组相比,LV-Lhx8组SKOV3细胞的增殖能力降低(P<0.01,图 2A、2C),而干扰Lhx8表达后细胞的增殖能力高于NC和WT组(P<0.01,图 2B、2D),提示Lhx8过表达可抑制SKOV3细胞的增殖。
|
图 2 过表达和干扰Lhx8后SKOV3细胞增殖能力的变化 Fig 2 Proliferation ability of SKOV3 cells transfected with LV-Lhx8 or Lhx8-siRNA A, C: Cell proliferation ability of SKOV3 cells transfected with lentivirus-mediated Lhx8 overexpression (LV-Lhx8) by EDU assay; B, D: Cell proliferation ability of SKOV3 cells transfected with Lhx8-targeted small interfering RNA (Lhx8-siRNA) by EDU assay; E: The cell number of SKOV3 cells transfected with LV-Lhx8 at different phases of cell cycle by flow cytometry; F: The cell number of SKOV3 cells transfected with Lhx8-siRNA at different phases of cell cycle by flow cytometry. **P < 0.01 vs LV-NC and WT groups in Fig 2C and 2E, and vs NC and WT groups in Fig 2D and 2F. n=3, x±s |
为了进一步验证Lhx8对于SKOV3细胞增殖的影响,我们利用流式细胞仪分析过表达或者干扰Lhx8后的细胞周期变化。LV-Lhx8转染的SKOV3细胞处于G0/G1期细胞数目的比例高于LV-NC和WT组(P<0.01,图 2E)。然而,采用siRNA干扰Lhx8表达后,SKOV3细胞处于S期的细胞数目的比例高于NC和WT组(P<0.01,图 2F)。提示Lhx8可通过增加进入G0/G1期的细胞数目抑制SKOV3细胞增殖。
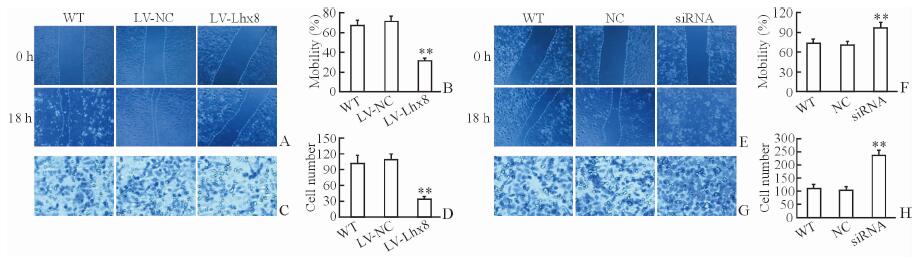
2.3 Lhx8对SKOV3细胞迁移和侵袭的影响相比LV-NC、WT组,过表达Lhx8抑制SKOV3细胞的迁移(P<0.01,图 3A、3B)和侵袭(P<0.01,图 3C、3D)。干扰Lhx8表达后则SKOV3细胞迁移(P<0.01,图 3E、3F)和侵袭能力高于NC和WT组(P<0.01,图 3G、3H)。
|
图 3 过表达或干扰Lhx8后SKOV3细胞迁移和侵袭能力的变化 Fig 3 Migration and invasion abilities of SKOV3 cells transfected with LV-Lhx8 or Lhx8-siRNA A, B: Migration ability by scratch assay in SKOV3 cells transfected with lentivirus-mediated Lhx8 overexpression (LV-Lhx8); C, D: Invasion ability by Transwell assay in the SKOV3 cells transfected with LV-Lhx8; E, F: Migration ability by scratch assay in SKOV3 cells transfected with Lhx8-targeted small interfering RNA (Lhx8-siRNA); G, H: Invasion ability by Transwell assay in the SKOV3 cells transfected with Lhx8-siRNA. Original magnification: ×100 (A, C, E, G). **P < 0.01 vs LV-NC and WT groups in Fig 3B and 3D, and vs NC and WT groups in Fig 3F and 3H. n=3, x±s |
2.4 Lhx8对MMP-2和MMP-9表达的影响
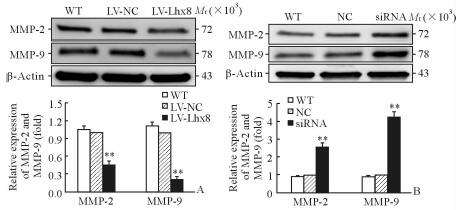
由于MMP在肿瘤侵袭转移中发挥关键作用,因此我们通过蛋白质印迹法检测了过表达或干扰Lhx8后MMP-2、MMP-9的表达。结果显示在LV-Lhx8组SKOV3细胞中MMP-2和MMP-9的表达低于LV-NC和WT组(P<0.01,图 4A)。然而用siRNA干扰Lhx8的表达后,MMP-2和MMP-9的蛋白表达均上调,与NC和WT组相比差异有统计学意义(P<0.01,图 4B)。
|
图 4 蛋白质印迹法检测过表达或干扰Lhx8后SKOV3细胞中MMP-2和MMP-9的蛋白表达 Fig 4 Expression of MMP-2 and MMP-9 in SKOV3 cells transfected with LV-Lhx8 or Lhx8-siRNA by Western blotting MMP: Matrix metalloproteinase. **P < 0.01 vs LV-NC and WT groups in Fig 4A, and vs NC and WT groups in Fig 4B. n=3, x±s |
3 讨论
LIM结构域被认为是蛋白质相互作用的功能模型,与其他蛋白结构域相互作用,介导功能复合物成员之间的特异性接触以及调节某些组成蛋白的活性[8]。研究表明,LIM基因在肿瘤发生、发展过程中扮演重要角色,而且多种LIM同源盒基因与不同类型的肿瘤发生有关[3-4]。Lhx8基因编码高保守LIM同源结构域(LIM-HD),LIM-HD基因在组织分化中发挥重要作用,尤其是神经组织和生殖细胞[7, 9-11]。Choi等[4]研究发现,小鼠Lhx8基因敲除不能生成原始卵泡,也不会出现从原始卵泡到成熟卵泡转化过程,最后导致卵母细胞特异基因Gdf9、Pou5f1和Nobox表达缺失。Li等[12]研究发现Lhx8能够抑制嗜铬细胞瘤PC12细胞的增殖,且过表达Lhx8能够使PC12细胞增殖停留在G1期。我们的研究结果显示过表达Lhx8能够使SKOV3细胞发育停留在G1/G0期,从而使细胞增殖能力降低,和Li等[12]的研究结果一致。
此外,细胞的增殖、迁移、侵袭能力是肿瘤恶性程度的主要评价指标。如Dormoy等[13]研究发现,LIM1的沉默能够减少肾细胞癌的迁移和侵袭,但是Lhx9不能直接影响细胞增殖和凋亡。我们的研究结果显示,Lhx8过表达抑制SKOV3细胞的增殖、迁移和侵袭,而干扰Lhx8表达则可促进SKOV3细胞增殖、迁移和侵袭;这与肾细胞癌中LIM1的研究结果[13]不同,但是与Lhx8对PC12细胞增殖能力的研究结果[12]一致。由此可见,虽然LIM1、Lhx8、Lhx9属于同一个家族,但是它们的作用机制并不完全一致。更详细的机制仍需要更多的体内外实验进一步阐明。
MMP通过激活蛋白1转录因子途径介导肿瘤细胞的迁移和侵袭[14]。在裸鼠体内使用siRNA或封闭抗体下调MMP-2的表达能够降低异种肿瘤体积并减少转移灶[15]。肿瘤发生转移后,可通过封闭MMP-2抑制肿瘤生长,这提示MMP-2能够抑制早期卵巢癌转移[16-17]。在体外研究中MMP-9主要参与路易斯肺癌的转移[18]。而且细丝蛋白A结合蛋白1(FILIP1L)可能通过减少β-catenin水平使WNT靶基因的转录水平下调,如MMP-9,最终抑制转移[19]。因此,我们通过蛋白质印迹法检测MMP-2和MMP-9蛋白的表达水平研究Lhx8与MMP-2、MMP-9之间的关系,结果显示在SKOV3细胞中过表达Lhx8减少了MMP-2、MMP-9的表达,干扰Lhx8后结果相反。
总之,Lhx8过表达能够抑制SKOV3细胞增殖、迁移、侵袭,反之,干扰Lhx8能够促进细胞增殖、迁移和侵袭,并且能够下调MMP-2和MMP-9的蛋白表达;这一研究结论将为进一步研究卵巢癌转移机制提供依据。
| [1] | TORRE L A, BRAY F, SIEGEL R L, FERLAY J, LORTET-TIEULENT J, JEMAL A. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65: 87–108. DOI: 10.3322/caac.21262 |
| [2] | KHALIL I, BREWER M A, NEYARAPALLY T, RUNOWICZ C D. The potential of biologic network models in understanding the etiopathogenesis of ovarian cancer[J]. Gynecol Oncol, 2010, 116: 282–285. DOI: 10.1016/j.ygyno.2009.10.085 |
| [3] | DAWID I B, TOYAMA R, TAIRA M. LIM domain proteins[J]. C R Acad Sci Ⅲ, 1995, 318: 295–306. |
| [4] | CHOI Y, BALLOW D J, XIN Y, RAJKOVIC A. Lim homeobox gene, Lhx8, is essential for mouse oocyte differentiation and survival[J]. Biol Reprod, 2008, 79: 442–449. DOI: 10.1095/biolreprod.108.069393 |
| [5] | ZHAO M, GUPTA V, RAJ L, ROUSSEL M, BEI M. A network of transcription factors operates during early tooth morphogenesis[J]. Mol Cell Biol, 2013, 33: 3099–3112. DOI: 10.1128/MCB.00524-13 |
| [6] | MORI T, YUXING Z, TAKAKI H, TAKEUCHI M, ISEKI K, HAGINO S, et al. The LIM homeobox gene, L3/Lhx8, is necessary for proper development of basal forebrain cholinergic neurons[J]. Eur J Neurosci, 2004, 19: 3129–3141. DOI: 10.1111/j.0953-816X.2004.03415.x |
| [7] | PFAFFL M W, GRAHAM W H, DEMPFLE L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR[J/OL]. Nucl Acids Res, 2002, 30: e36. doi: 10.1093/nar/30.9.e36. |
| [8] | DAWID I B, BREEN J J, TOYAMA R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions[J]. Trends Genet, 1998, 14: 156–162. DOI: 10.1016/S0168-9525(98)01424-3 |
| [9] | RICHTER K, PINTO DO O P, HÄGGLUND A C, WAHLIN A, CARLSSON L. Lhx2 expression in hematopoietic progenitor/stem cells in vivo causes a chronic myeloproliferative disorder and altered globin expression[J]. Haematologica, 2003, 88: 1336–1347. |
| [10] | JUNG S, JEONG D, KIM J, YI L, KOO K, LEE J, et al. Epigenetic regulation of the potential tumor suppressor gene, hLHX6.1, in human cervical cancer[J]. Int J Oncol, 2011, 38: 859–869. |
| [11] | DIETRICH D, LESCHE R, TETZNER R, KRISPIN M, DIETRICH J, HAEDICKE W, et al. Analysis of DNA methylation of multiple genes in microdissected cells from formalin-fixed and paraffin-embedded tissues[J]. J Histochem Cytochem, 2009, 57: 477–489. DOI: 10.1369/jhc.2009.953026 |
| [12] | LI H, QIN J, JIN G, ZOU L, SHI J, HAN X, et al. Overexpression of Lhx8 inhibits cell proliferation and induces cell cycle arrest in PC12 cell line[J]. In Vitro Cell Dev Biol Anim, 2015, 51: 329–335. DOI: 10.1007/s11626-014-9838-y |
| [13] | DORMOY V, BÉRAUD C, LINDNER V, THOMAS L, COQUARD C, BARTHELMEBS M, et al. LIM-class homeobox gene Lim1, a novel oncogene in human renal cell carcinoma[J]. Oncogene, 2011, 30: 1753–1763. DOI: 10.1038/onc.2010.557 |
| [14] | BENBOW U, BRINCKERHOFF C E. The AP-1 site and MMP gene regulation: what is all the fuss about?[J]. Matrix Biol, 1997, 15(8/9): 519–526. |
| [15] | WESTERMARCK J, KÄHÄRI V M. Regulation of matrix metalloproteinase expression in tumor invasion[J]. FASEB J, 1999, 13: 781–792. |
| [16] | KENNY H A, LENGYEL E. MMP-2 functions as an early response protein in ovarian cancer metastasis[J]. Cell Cycle, 2009, 8: 683–638. DOI: 10.4161/cc.8.5.7703 |
| [17] | HU X, LI D, ZHANG W, ZHOU J, TANG B, LI L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion[J]. Arch Gynecol Obstet, 2012, 286: 1537–1543. DOI: 10.1007/s00404-012-2456-6 |
| [18] | CHOU C H, TENG C M, TZEN K Y, CHANG Y C, CHEN J H, CHENG J C. MMP-9 from sublethally irradiated tumor promotes Lewis lung carcinoma cell invasiveness and pulmonary metastasis[J]. Oncogene, 2012, 31: 458–468. DOI: 10.1038/onc.2011.240 |
| [19] | KWON M, LEE S J, WANG Y, RYBAK Y, LUNA A, REDDY S, et al. Filamin A interacting protein 1-like inhibits WNT signaling and MMP expression to suppress cancer cell invasion and metastasis[J]. Int J Cancer, 2014, 135: 48–60. DOI: 10.1002/ijc.v135.1 |
 2017, Vol. 38
2017, Vol. 38


