2. 第二军医大学国际合作肿瘤研究所, 上海 200433
2. International Joint Cancer Institute, Second Military Medical University, Shanghai 200433, China
目前,对于肝脏中具有干性的细胞(如肝前体细胞)已有不少研究[1-3],但对于肝脏中真正的肝干细胞则缺乏认识。虽然不同研究所报道的肝干性细胞的表型和分子特征存在着很大的差异,但它们大都具有一个共同的特征,即表达细胞角蛋白19(cytokeratin 19, CK19)[4]。CK19一般在胰腺和胆管中表达[5]。基于这一现象,结合本课题组前期有关肝前体细胞研究的发现,本研究提出了“肝脏中的CK19+细胞谱系中可能包含真正的肝干细胞(authentic liver stem cell)”的科学假设。为了证实这一假设,本研究采用Cre/loxP体系对CK19+细胞进行谱系示踪,并结合肝损伤模型分析CK19+细胞的分化特性。
1 材料和方法 1.1 仪器、试剂与动物BA210-T型光学显微镜购于麦克奥迪实业集团有限公司,ECLIPSE TE2000-U型荧光显微镜和摄影系统购于日本尼康公司,80-2型台式低速离心机购于上海医疗器械(集团)有限公司手术器械厂,CT15RE型超低温高速离心机购于日本日立公司,DYY Ⅲ 31C型凝胶电泳仪购于北京六一生物科技有限公司,ZELBoCXR凝胶成像系统购于美国Bio-Rad公司,PIPETMAN Neo微量加样器购于美国Gilson公司,手术器械购于强生(上海)医疗器械有限公司,ProFlex PCR仪购于美国Life公司,Heal Force SMART Ultra-pure超纯水仪购于力康生物医疗科技控股有限公司,CM1850型冰冻切片机、RM2235型石蜡切片机均购于德国Leica公司,158105型防脱载玻片和盖玻片购于江苏世泰实验器材有限公司,RNase-free离心管、PCR管均购于美国Axygen公司。异丙醇、无水乙醇购于国药集团化学试剂有限公司;四氯化碳(carbon tetrachloride,CCl4)购于美国Sigma公司,按体积比1:10溶于橄榄油中使用。他莫昔芬(tamoxifen, TM)、DAPI购于美国Sigma公司,苏木精染液、伊红染液购于上海碧云天生物技术有限公司;绿色荧光蛋白(GFP)一抗(A11120)、驴抗兔荧光二抗(A21206) 和驴抗鼠荧光二抗(A10037) 均购于美国Invitrogen公司,CK19一抗(A3190) 购于美国Abbomax公司,白蛋白(albumin,ALB)一抗(Ab19194) 购于英国Abcam公司,其中CK19一抗和ALB一抗按1:200稀释比用1% BSA稀释;GFP一抗(A11120)、驴抗兔荧光二抗和驴抗鼠荧光二抗按1:500稀释比用1% BSA稀释。含3,5-二乙氧基羰基-1,4-二氢-2,4,6-三甲基吡啶(3,5-diethoxycarbonyl-1,4-dihydrocollidine,DDC)饲料购于海军医学研究所动物中心。CK19CreERT小鼠购于上海南方模式动物研究中心[动物许可证号:SCXK(沪)2014-0002];Rosa26-GFP小鼠从美国杰克逊实验室(the Jackson Laboratory)引进,与CK19CreERT小鼠同为C57BL/6品系。在Rosa26-GFP小鼠的Rosa26启动子与GFP之间插入loxP-stop-loxP结构(Rosa26-loxP-stop-loxP-GFP),正常情况下该小鼠不表达GFP,当存在Cre酶时能够重组或切除stop位点使GFP表达;在CK19CreERT小鼠的CK19基因位点后面加入与雌激素受体配体结合区融合的Cre重组酶——CreERT,正常情况下小鼠体内的Cre重组酶位于胞质中,摄入TM后其可进入核内发挥作用。
1.2 CK19CreERT/Rosa26-GFP双转基因小鼠的鉴定CK19CreERT小鼠和Rosa26-GFP小鼠饲养于第二军医大学实验动物中心的SPF级动物房,自由进食,昼夜节律(7:00~19:00光照,19:00~次日7:00黑暗)。CK19CreERT小鼠与Rosa26-GFP小鼠杂交方法:将鉴定结果阳性的子代3个月龄CK19CreERT小鼠与3个月龄Rosa26-GFP小鼠进行配对,并给予高热量饮食,记录预产时间。得到的子代杂交鼠于出生后2周剪尾抽提DNA并行PCR鉴定,建立CK19CreERT/Rosa26-GFP双转基因小鼠品系。所有小鼠使用前均需要经过PCR鉴定。小鼠品系PCR鉴定步骤如下:(1) 抽提DNA。小鼠于出生后2周在无菌环境下剪尾,消毒后将组织放入1.5 mL离心管中,每管加入混合消化液250 μL;55 ℃孵育,间或震荡,持续5 h以上确保完全消化;加入250 μL异丙醇、氯仿和苯酚的混合液,漩涡震荡,15 000×g离心12 min;吸取上清至新的1.5 mL离心管中,加入异丙醇300 μL,震荡后可见絮状沉淀;15 000×g离心5 min,保留沉淀,加入70%乙醇700 μL洗涤;15 000×g离心5 min,去除上清,倒置晾干。将抽提所得DNA溶于50 μL高压灭菌的ddH2O中,-20 ℃保存备用。(2) PCR反应。反应条件为94 ℃变性30 s,58 ℃退火30 s,72 ℃延伸1 min,共32个循环。引物序列为CK19CreERT F 5′-GTT CTT GCG AAC CTC ATC ACT C-3′,R 5′-GCA GAA TCG CCA GGA ATT GAC C-3′;Rosa26 F 5′-AAC CAG AAG TGG CAC CTG AC-3′,R 5′-GGC ATT AAA GCA GCG TAT CC-3′。
1.3 杂交小鼠报告系统检测CK19CreERT/Rosa26-GFP小鼠于出生后8周经腹腔注射4 mg TM,2 d后颈椎脱臼处死,取肝并切成适当大小放入生理盐水中洗净,后使用自制包埋框加入OCT包埋,-20 ℃冻存后连续切片,切片厚度5 μm。4%多聚甲醛溶液固定5 min,PBS漂洗15 min,直接滴加DAPI封片剂封片后在荧光显微镜下观察绿色荧光分布情况。之后用红色荧光标记的二抗检测CK19。1% BSA封闭切片30 min,加一抗,37 ℃温箱孵育1 h,PBS漂洗15 min;加入红色荧光标记的二抗,37 ℃孵育30 min,PBS漂洗15 min,使用DAPI封片剂封片后在荧光显微镜下观察并拍照。
1.4 DDC肝脏损伤模型和CCl4肝脏损伤模型的构建DDC肝脏损伤模型构建方法:CK19CreERT/Rosa26-GFP小鼠于出生后8周注射4 mg TM诱导Cre酶入核,第9周开始实验组小鼠给予DDC饲料喂养造成慢性肝脏损伤,对照组一直给予正常小鼠饲料,持续喂养6周后取肝组织包埋切片和染色。
CCl4肝脏损伤模型构建方法:CK19CreERT/Rosa26-GFP小鼠于出生后8周注射4 mg TM诱导Cre酶入核,第9周称取实验组小鼠体质量,并编号。建立表格记载,按1 μL/g体质量计算各小鼠所需的CCl4注射量,经腹腔进行单次注射CCl4诱导肝损伤。1周后取肝组织行包埋冰冻切片,荧光显微镜下观察GFP+细胞的分布情况,免疫荧光检测肝细胞标志物ALB。对照组小鼠在TM注射后1周按1 μL/g体质量经腹腔单次注射橄榄油,其余操作同实验组。
1.5 免疫荧光染色小鼠颈椎脱臼处死,取肝并切成适当大小放入生理盐水中洗净,后使用自制包埋框加入OCT包埋,-20 ℃冻存后切片,切片厚度5 μm。用4%多聚甲醛溶液固定5 min,PBS漂洗15 min,直接滴加DAPI封片剂封片后在荧光显微镜下观察绿色荧光分布情况。如需行免疫荧光染色,则用疏水性笔在组织周围画圈,节约抗体用量并防止抗体流失;1% BSA封闭30 min,加一抗,37 ℃温箱避光孵育1 h;PBS漂洗15 min,加入红色荧光标记的二抗,37 ℃避光孵育30 min;PBS漂洗15 min;使用DAPI封片剂封片,在荧光显微镜下观察并拍照。
1.6 H-E染色颈椎脱臼法处死小鼠后从腹中线切开,取出肝脏并切成合适大小的组织块放入生理盐水中洗净;将组织块编号,放入装有4%多聚甲醛溶液的15 mL离心管中固定24 h;弃掉固定液,75%乙醇洗1遍后放入装有75%乙醇的15 mL离心管中,4 ℃长期保存。将组织块修理成适合包埋的大小,经85%、95%、100%乙醇梯度脱水各30 min,二甲苯透明20 min,之后依序放入58 ℃ 1号、2号、3号蜡缸中各1 h使其浸泡完全;取出组织块,使用包埋机包埋。室温放置过夜使蜡块完全凝固,修理蜡块;将修理好的蜡块4 ℃冷冻20 min后切片,厚度为2 μm,之后漂于40 ℃漂片机内,使用防脱载玻片捞片;烤片机40 ℃烘烤过夜,收集至切片盒长期保存。将切片置于烤片机上60 ℃烤25 min使蜡完全溶解,依次加入二甲苯1、二甲苯2、二甲苯/乙醇各25 min脱蜡处理;将脱蜡后的切片依次放入100%、90%、80%、70%、50%乙醇中各5 min,后放入蒸馏水中2 min。H-E染色:苏木精染色5~10 min,流水冲洗15 min返蓝,双蒸水浸泡数秒,95%乙醇浸泡30 s;伊红染色30 s至2 min,镜检观察。将需要留用的切片依次加入50%、70%、80%、90%、100%乙醇各5 min梯度脱水,之后放入二甲苯/乙醇、二甲苯2、二甲苯1中各15 min透明。取出后滴加1滴中性树胶,盖上盖玻片并轻轻压去气泡,室温放置晾干后放入切片盒中长期保存。
1.7 统计学处理应用GraphPad Prism 5软件进行数据分析。所有实验均重复3次,数据以x±s表示,组间比较采用t检验。检验水准(α)为0.05。
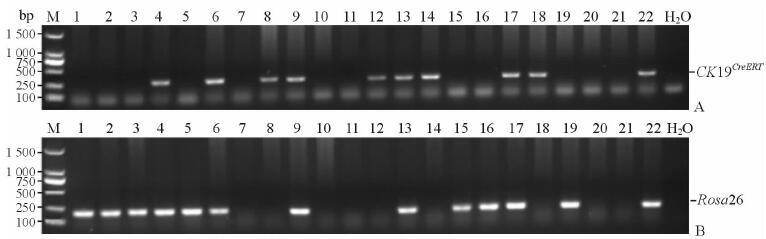
2 结果 2.1 杂交后小鼠基因型鉴定Rosa26阳性条带大小为199 bp,CK19CreERT阳性条带大小为300 bp。通过对CK19CreERT小鼠与Rosa26-GFP小鼠杂交后得到的22只子代小鼠进行鉴定,结果(图 1)发现,4、6、8、9、12、13、14、17、18和22号小鼠为CK19CreERT阳性小鼠,而1、2、3、4、5、6、9、13、15、16、17、19和22号小鼠为Rosa26阳性小鼠。可见22只子代小鼠中4、6、9、13、17和22号小鼠为CK19CreERT和Rosa26双阳性小鼠,即为CK19CreERT/Rosa26-GFP双转基因小鼠。
|
图 1 杂交后子代小鼠品系鉴定 Fig 1 Genotyping of offsprings of hybrid mice using PCR A: 300 bp PCR products were detected in tail DNA samples from CK19CreERT mice; B: 199 bp PCR products were detected in tail DNA samples from Rosa26-GFP mice. The far left lanes in both figures were DNA markers with sizes of all bands (in base pairs). The far right lanes (labeled as H2O) were negative controls. No.1-22 represented hybrid mice |
2.2 CK19+细胞GFP标记情况检测
注射TM后, 小鼠肝脏内出现特异性的绿色荧光,荧光较强,并且主要分布在门管区(图 2)。免疫荧光检测结果见图 3。在CK19CreERT/Rosa26-GFP小鼠肝脏中CK19+细胞的GFP标记效率为(53.9±8.6)%。

|
图 2 未损伤杂交小鼠注射TM后的肝脏荧光检测 Fig 2 Detection of liver fluorescence after TM injection in non-injured CK19CreERT/Rosa26-GFP mice A: Nuclear staining with DAPI (blue); B: GFP expression in the liver of non-injured CK19CreERT/Rosa26-GFP mice; C: DAPI staining (blue) merged with the GFP immunofluorescence. GFP: Green fluorescent protein; DAPI: 4',6-Diamidino-2-phenylindole. Scale bar=100 μm |
|
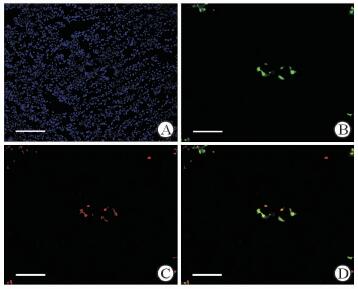
图 3 未损伤杂交小鼠模型的GFP表达与CK19免疫荧光染色 Fig 3 GFP expression and CK19 immunofluorescence staining in non-injured CK19CreERT/Rosa26-GFP mice A: Nuclear staining with DAPI (blue); B: GFP expression in the liver of non-injured CK19CreERT/Rosa26-GFP mice; C: Immunofluorescence staining using CK19 antibody (red); D: CK19 staining (red) merged with GFP immunofluorescence. GFP: Green fluorescent protein. Scale bar=100 μm |
2.3 DDC诱导肝脏损伤模型的增生性小胆管中出现GFP+细胞
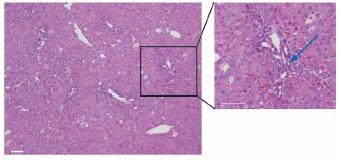
对照组小鼠肝脏冰冻切片荧光显微镜观察可见GFP+细胞分布在门管区(图 4),GFP+胆管细胞比例为(53.6±4.8)%。DDC喂养后小鼠肝脏H-E染色可见肝组织出现炎症细胞浸润,汇管区未成熟小胆管数量增多(图 5);肝组织冰冻切片荧光显微镜观察可见肝脏中出现增生性小胆管,其中含有GFP+细胞,荧光强度较好,胆管结构完整,而检测胆管标志物CK19结果显示红色荧光与绿色荧光吻合度很好(图 6)。DDC肝脏损伤模型组小鼠GFP+胆管细胞比例为(63.5±6.3)%,高于对照组(P < 0.05)。

|
图 4 DDC对照组小鼠肝脏中GFP的表达 Fig 4 GFP expression in liver of mice in DDC control group A: Nuclear staining with DAPI (blue); B: GFP expression in the liver of the mice in DDC control group; C: DAPI staining (blue) merged with the GFP immunofluorescence. DDC: 3,5-Diethoxycarbonyl-1,4-dihydrocollidine; GFP: Green fluorescent protein; DAPI: 4',6-Diamidino-2-phenylindole. Scale bar=100 μm |
|
图 5 DDC肝脏损伤模型组小鼠的肝脏组织学改变(H-E染色) Fig 5 Histological changes in liver of DDC-induced liver injury mouse model (H-E staining) DDC: 3,5-Diethoxycarbonyl-1,4-dihydrocollidine. Reactive hyperplastic changes were found in biliary epithelial cells. The number of immature small bile ducts was increased (blue arrow) and gathered around the inflammatory cells. Scale bar=100 μm |
|
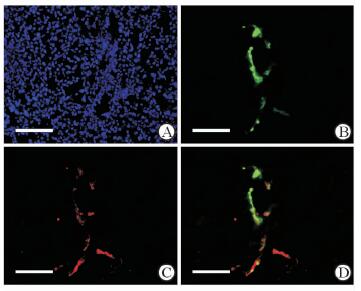
图 6 DDC肝脏损伤模型组小鼠肝脏中GFP表达和CK19免疫荧光染色 Fig 6 GFP expression and CK19 immunofluorescence analysis in liver of DDC-induced liver injury mouse model A: Nuclear staining with DAPI (blue); B: GFP expression in the liver of DDC-induced liver injury mouse model; C: Immunofluorescence staining using CK19 antibody (red); D: CK19 staining (red) merged with the GFP immunofluorescence. DDC: 3,5-Diethoxycarbonyl-1,4-dihydrocollidine; GFP: Green fluorescent protein. Scale bar=100 μm |
2.4 CCl4诱导的肝脏损伤模型中CK19+细胞可以分化为肝细胞
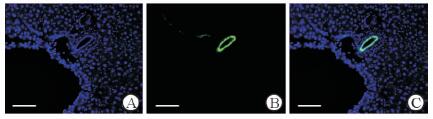
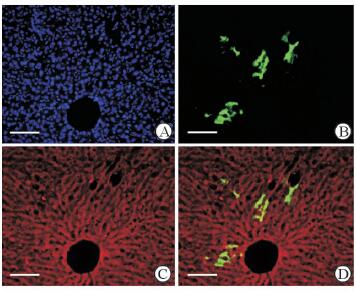
对照组小鼠肝脏冰冻切片荧光显微镜观察可见GFP+细胞局限在门管区胆管上皮周围,肝实质有极少量GFP+肝细胞(图 7),比例为(0.008±0.003)%。经CCl4诱导肝细胞损伤后,小鼠肝实质中GFP+肝细胞增多,占肝实质细胞总量的(0.15±0.02)%,高于对照组(P < 0.01),它们呈簇状分布在肝小叶内;免疫荧光检测结果示GFP+细胞表达成熟肝细胞特异性标志物ALB(图 8)。
|
图 7 CCl4对照组小鼠肝脏中GFP的表达 Fig 7 GFP expression in liver of mice in CCl4 control group A: Nuclear staining with DAPI (blue); B: GFP expression in the liver of the mice in the CCl4 control group; C: DAPI staining (blue) merged with the GFP immunofluorescence. GFP: Green fluorescent protein; DAPI: 4',6-Diamidino-2-phenylindole; CCl4: Carbon tetrachloride. Scale bar=100 μm |
|
图 8 CCl4肝脏损伤模型组小鼠肝脏中GFP和ALB的表达 Fig 8 GFP and ALB expression in liver of CCl4-induced liver injury mouse model A: Nuclear staining with DAPI (blue); B: GFP expression in the liver of the mice in the CCl4-induced liver injury model group; C: Immunofluorescence staining using ALB antibody (red); D: ALB staining (red) merged with the GFP immunofluorescence. CCl4: Carbon tetrachloride; GFP: Green fluorescent protein; ALB: Albumin; DAPI: 4',6-Diamidino-2-phenylindole. Scale bar=100 μm |
3 讨论
肝干细胞在普通生理情况下处于静息状态,并不会发生增殖和分化[6],所以肝干细胞的相关研究往往需要借助肝脏损伤模型。有研究发现在啮齿动物肝脏中存在卵圆细胞,这种细胞具有双向分化潜能,既能分化为肝细胞[7-9],又能分化为胆管细胞[10-12],而它们的出现又与肝脏损伤有关[13]。目前常被用来研究肝干细胞的动物模型主要有急性和慢性肝损伤模型。急性肝损伤模型有以下几种:(1) 肝2/3切除模型[14],能诱导肝再生;已有研究证明肝再生过程中主要是肝细胞发挥作用[11],故该模型用来进行肝干细胞研究比较困难,需要外源性给予药物或基因打靶抑制肝细胞的自我更新,从而使干细胞获得增殖优势。(2) CCl4单次大剂量注射模型[15],其损伤肝脏的机制主要是参与自由基代谢,CCl4进入肝脏后能经P450催化激活产生过氧自由基,与细胞膜上的磷脂反应使细胞膜和细胞器受损,还能通过与肝内大分子蛋白质结合影响代谢[16]。CCl4主要损伤肝细胞,能使肝细胞坏死并伴随明显的炎症反应。常用的慢性肝损伤模型有DDC饲料长期喂养模型[17]。研究发现DDC肝脏损伤模型能够引起原始小胆管细胞的持续增殖,即非典型性胆管细胞增殖(ADP),胆管谱系的细胞在门静脉周围增殖形成原始小管并修复受损的肝组织,ADP起源于肝小叶的门管区,之后向肝小叶扩散,分化成为肝细胞样的细胞[13]。本研究采用DDC和CCl4两种肝脏损伤模型来研究CK19+细胞的分化情况。
Cre/loxP介导的基因重组允许我们在胚胎发育和组织再生期间进行基因功能和细胞谱系分析[18-20]。CK19CreERT小鼠是在CK19基因位点后面加入与雌激素受体配体结合区融合的Cre重组酶所构建的,正常情况下小鼠体内的Cre酶无法发挥活性,摄入TM之后使其进入核内发挥作用。当没有TM存在时只能在少于1%的胃肠上皮细胞中检测到Cre酶活性,而在胰腺和肝脏的上皮样组织中完全检测不到。腹腔注射TM能够诱导动物胰腺管、肝内胆管、胃和肠道上皮细胞基因重组,并且重组程度与注射的TM剂量成正比。有研究表明CK19CreERT小鼠在注射TM后能够使肠道干细胞被Cre酶标记,并且其子代细胞也持续带有该标记[21],提示我们能够使用该小鼠进行肝脏等其他器官组织干细胞的示踪研究。
CK19基因编码一种中间丝蛋白[22],该蛋白在外胚层多种细胞中表达,并且持续表达至胚胎后期和产后阶段[22-25]。CK19在成人胰腺腺管中高表达[26],但在腺泡和胰岛中不表达[27]。同样CK19在中间型肝胆细胞中高表达而在成熟肝细胞中不表达[28]。有研究提示胰腺腺管和中间型肝胆细胞可能起到组织干细胞的功能,维持组织再生[29-30]。CK19CreERT小鼠能够对这些表达CK19的细胞进行标记,并且在组织再生过程中显示这些细胞的命运。但该品系小鼠自身不带有荧光标记,因此需要将它与Rosa26-GFP小鼠杂交得到CK19CreERT/Rosa26-GFP小鼠,并在小鼠出生后8周经腹腔注射一定剂量的TM诱导胞质中的Cre酶入核发挥作用,使小鼠体内CK19+的细胞带上GFP标记,从而直接行荧光显微镜检测。
本研究首先利用C57BL/6品系的CK19CreERT小鼠与Rsoa26-GFP小鼠杂交,获得CK19CreERT/Rosa26-GFP双转基因小鼠。在此基础上应用TM实现了肝脏中CK19+细胞的稳定性遗传标记(即标记上GFP),进而采用肝脏组织冰冻切片结合免疫荧光染色明确该标记细胞在肝脏中的空间定位为门管区。随后,利用CK19+细胞已被遗传标记的CK19CreERT/Rosa26-GFP小鼠制备DDC肝损伤模型和CCl4肝损伤模型,探讨肝脏中GFP所标记的CK19+细胞的分化情况。DDC肝损伤小鼠模型可以发生胆管损伤,并可出现损伤修复,表现为胆管反应的发生[13]。CCl4肝损伤的小鼠模型可以发生肝细胞的损伤,并可出现肝细胞的修复[15]。本研究成功构建了基于CK19CreERT/Rosa26-GFP小鼠的DDC肝损伤模型和CCl4肝损伤模型,并发现在DDC损伤模型小鼠中增生性小胆管内出现了GFP+细胞,且这些GFP+细胞表达CK19,表明存在于这些增生性胆管中被标记的GFP+细胞即是CK19+细胞,也提示基于GFP的表达可以示踪肝脏中CK19+细胞的分化去向;而在CCl4肝损伤模型小鼠中发现,肝小叶中存在GFP+细胞,且这些GFP+细胞表达成熟肝细胞标志物ALB,表明这些ALB+细胞来源于谱系示踪标记的GFP+细胞,也提示谱系示踪标记的GFP+细胞中存在有肝向分化潜能的细胞类型。
综上所述,小鼠肝脏内的CK19+细胞群体中存在有具有肝向分化潜能的肝前体细胞,这一发现为肝干细胞的识别鉴定提供了新线索。
| [1] | ZAJICEK G, OREN R, WEINREB M J. The streaming liver[J]. Liver, 1985, 5: 293–300. |
| [2] | FURUYAMA K, KAWAGUCHI Y, AKIYAMA H, HORIGUCHI M, KODAMA S, KUHARA T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine[J]. Nat Genet, 2011, 43: 34–41. DOI: 10.1038/ng.722 |
| [3] | WANG B, ZHAO L, FISH M, LOGAN C Y, NUSSE R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver[J]. Nature, 2015, 524: 180–185. DOI: 10.1038/nature14863 |
| [4] | LOMBARD C A, PRIGENT J, SOKAL E M. Human liver progenitor cells for liver repair[J]. Cell Med, 2013, 5: 1–16. DOI: 10.3727/215517913X666459 |
| [5] | JAIN R, FISCHER S, SERRA S, CHETTY R. The use of Cytokeratin 19(CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver[J]. Appl Immunohistochem Mol Morphol, 2010, 18: 9–15. DOI: 10.1097/PAI.0b013e3181ad36ea |
| [6] | THEISE N D. Liver stem cells[J]. Cytotechnology, 2003, 41: 139–144. DOI: 10.1023/A:1024826823194 |
| [7] | FARIS R A, HIXSON D C. Selective proliferation of chemically altered rat liver epithelial cells following hepatic transplantation[J]. Transplantation, 1989, 48: 87–92. DOI: 10.1097/00007890-198907000-00021 |
| [8] | YASUI O, MIURA N, TERADA K, KAWARADA Y, KOYAMA K, SUGIYAMA T. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver[J]. Hepatology, 1997, 25: 329–334. |
| [9] | SONG S, WITEK R P, LU Y, CHOI Y K, ZHENG D, JORGENSEN M, et al. Ex vivo transduced liver progenitor cells as a platform for gene therapy in mice[J]. Hepatology, 2004, 40: 918–924. DOI: 10.1002/(ISSN)1527-3350 |
| [10] | LENZI R, LIU M H, TARSETTI F, SLOTT P A, ALPINI G, ZHAI W R, et al. Histogenesis of bile duct-like cells proliferating during ethionine hepatocarcinogenesis. Evidence for a biliary epithelial nature of oval cells[J]. Lab Invest, 1992, 66: 390–402. |
| [11] | DUNCAN A W, DORRELL C, GROMPE M. Stem cells and liver regeneration[J]. Gastroenterology, 2009, 137: 466–481. DOI: 10.1053/j.gastro.2009.05.044 |
| [12] | SACKETT S D, LI Z, HURTT R, GAO Y, WELLS R G, BRONDELL K, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice[J]. Hepatology, 2009, 49: 920–929. DOI: 10.1002/hep.22705 |
| [13] | PREISEGGER K H, FACTOR V M, FUCHSBICHLER A, STUMPTNER C, DENK H, THORGEIRSSON S S. Atypical ductular proliferation and its inhibition by transforming growth factor beta1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease[J]. Lab Invest, 1999, 79: 103–109. |
| [14] | BOYCE S, HARRISON D. A detailed methodology of partial hepatectomy in the mouse[J]. Lab Anim (NY), 2008, 37: 529–532. DOI: 10.1038/laban1108-529 |
| [15] | LIU Y, ZHOU Q, HE X S, SONG L M, CHEN L, JIAO W J, et al. Genetic variants in ERBB4 is associated with chronic hepatitis B virus infection[J]. Oncotarget, 2016, 7: 4981–4992. |
| [16] | RECKNAGEL R O, GLENDE E A Jr, DOLAK J A, WALLER R L. Mechanisms of carbon tetrachloride toxicity[J]. Pharmacol Ther, 1989, 43: 139–154. DOI: 10.1016/0163-7258(89)90050-8 |
| [17] | KIM K H, SUNG H J, LEE W R, AN H J, KIM J Y, PAK S C, et al. Effects of melittin treatment in cholangitis and biliary fibrosis in a model of xenobiotic-induced cholestasis in mice[J]. Toxins (Basel), 2015, 7: 3372–3387. DOI: 10.3390/toxins7093372 |
| [18] | BRANDA C S, DYMECKI S M. Talking about a revolution:the impact of site-specific recombinases on genetic analyses in mice[J]. Dev Cell, 2004, 6: 7–28. DOI: 10.1016/S1534-5807(03)00399-X |
| [19] | LEWANDOSKI M. Conditional control of gene expression in the mouse[J]. Nat Rev Genet, 2001, 2: 743–755. DOI: 10.1038/35093537 |
| [20] | SAUER B, HENDERSON N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1[J]. Proc Natl Acad Sci USA, 1988, 85: 5166–5170. DOI: 10.1073/pnas.85.14.5166 |
| [21] | MEANS A L, XU Y, ZHAO A, RAY K C, GU G. A CK19CreERT knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs[J]. Genesis, 2008, 46: 318–323. DOI: 10.1002/dvg.v46:6 |
| [22] | MOLL R, FRANKE W W, SCHILLER D L, GEIGER B, KREPLER R. The catalog of human cytokeratins:patterns of expression in normal epithelia, tumors and cultured cells[J]. Cell, 1982, 31: 11–24. DOI: 10.1016/0092-8674(82)90400-7 |
| [23] | BOSCH F X, LEUBE R E, ACHTSTATTER T, MOLL R, FRANKE W W. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ[J]. J Cell Biol, 1988, 106: 1635–1648. DOI: 10.1083/jcb.106.5.1635 |
| [24] | LANE E B, HOGAN B L, KURKINEN M, GARRELS J I. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo[J]. Nature, 1983, 303: 701–704. DOI: 10.1038/303701a0 |
| [25] | QUINLAN R A, SCHILLER D L, HATZFELD M, ACHTSTATTER T, MOLL R, JORCANO J L, et al. Patterns of expression and organization of cytokeratin intermediate filaments[J]. Ann N Y Acad Sci, 1985, 455: 282–306. DOI: 10.1111/nyas.1985.455.issue-1 |
| [26] | DERAMAUDT T B, SACHDEVA M M, WESCOTT M P, CHEN Y, STOFFERS D A, RUSTGI A K. The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter[J]. J Biol Chem, 2006, 281: 38385–38395. DOI: 10.1074/jbc.M605891200 |
| [27] | BREMBECK F H, MOFFETT J, WANG T C, RUSTGI A K. The keratin 19 promoter is potent for cell-specific targeting of genes in transgenic mice[J]. Gastroenterology, 2001, 120: 1720–1728. DOI: 10.1053/gast.2001.24846 |
| [28] | NISHIKAWA Y, TOKUSASHI Y, KADOHAMA T, NISHIMORI H, OGAWA K. Hepatocytic cells form bile duct-like structures within a three-dimensional collagen gel matrix[J]. Exp Cell Res, 1996, 223: 357–371. DOI: 10.1006/excr.1996.0091 |
| [29] | XU G, KANETO H, LOPEZ-AVALOS M D, WEIR G C, BONNER-WEIR S. GLP-1/exendin-4 facilitates beta-cell neogenesis in rat and human pancreatic ducts[J]. Diabetes Res Clin Pract, 2006, 73: 107–110. DOI: 10.1016/j.diabres.2005.11.007 |
| [30] | DORRELL C, GROMPE M. Liver repair by intra-and extrahepatic progenitors[J]. Stem Cell Rev, 2005, 1: 61–64. DOI: 10.1385/SCR:1:1 |
 2017, Vol. 38
2017, Vol. 38


