代谢手术作为2型糖尿病的治疗方法之一,可影响胃肠道多种激素的水平[1-2],代谢手术在糖尿病发生、发展过程中对激素水平及其受体功能有着重要影响。因此阐明其影响机制对临床治疗糖尿病药物的探索具有重要意义。代谢手术“后肠假说”的代表激素是胰高血糖素样肽1(glucagon-like peptide 1,GLP-1),它可以激活环磷酸腺苷/蛋白激酶A(cAMP/PKA)通路。该信号通路参与调控胰岛β细胞分泌胰岛素,从而维持血糖稳定[3-4];袖状胃切除术具有一定的降血糖效果,主要是通过降低胃底分泌的胃饥饿素(ghrelin)来发挥作用[5-6]。有研究表明ghrelin通过生长激素促泌素受体1α(growth hormone secretagogue receptor 1α,GHSR-1α)竞争性抑制GLP-1刺激胰岛β细胞的促胰岛素分泌效应[7]。但ghrelin对竞争性抑制GLP-1促胰岛素分泌效应的竞争性抑制作用的具体机制尚不清楚,其是否与cAMP/PKA通路直接关联也无明确的研究报道,因此,本研究旨在探索ghrelin是否通过cAMP/PKA通路竞争性抑制GLP-1对胰岛细胞分泌胰岛素的影响。
1 材料和方法 1.1 实验动物及材料5只8~10周龄250~300 g SPF级成年雄性SD大鼠由第二军医大学实验动物中心提供[动物生产许可证号:SCXK(沪)2013-0016)]。GLP-1、GHSR-1α拮抗剂生长激素释放肽6(D-Lys3-GHRP-6)、腺苷酸环化酶激动剂毛喉素(forskolin)、PKA激动剂(6-Phe-cAMP)和ghrelin均购自美国Sigma公司。RPMI 1640培养基(货号:R10-040-CV,美国Corning公司);大鼠胰岛素ELISA检测试剂盒(货号:10-1250-01,瑞典Mercodia公司);cAMP ELISA检测试剂盒(货号:20161226,南京建成生物工程研究所)。RT-2100C型酶标分析仪(深圳雷杜生命科学股份有限公司)。
1.2 双硫腙(DTZ)染色分离、纯化胰岛[8],体外培养48 h进行DTZ染色。将10 mg DTZ溶于10 mL DMSO中,用pH 7.8的Hank’s液1:1 000稀释,经0.22 μm孔径滤膜过滤,然后与胰岛混合,室温染色10 min后采用光学显微镜观察胰岛形态。
1.3 吖啶橙/碘化丙啶(AO/PI)染色用Hank’s液配制储存液:AO 670 μmol/L、PI 750 μmol/L,4 ℃避光保存。将0.01 mL AO、1 mL PI储存液混合,用Hank’s液稀释10倍,0.22 μm孔径滤膜过滤,与胰岛混合10 min后,于荧光显微镜下观察胰岛生长状态。
1.4 实验分组与处理在显微镜下挑选形态饱满、直径为100 μm的胰岛。每只大鼠挑选60个胰岛,随机分入6组并接受不同处理。S0组(8.3 mmol/L葡萄糖溶液)、S1组(8.3 mmol/L葡萄糖溶液+10 nmol/L[9] GLP-1)、S2组(8.3 mmol/L葡萄糖溶液+10 nmol/L GLP-1+10 nmol/L[9]ghrelin)、S3组[8.3 mmol/L葡萄糖溶液+10 nmol/L GLP-1+10 nmol/L ghrelin+1 μmol/L[9]D-Lys3-GHRP-6]、S4组(8.3 mmol/L葡萄糖溶液+10 nmol/L GLP-1+10 nmol/L ghrelin+5 μmol/L[10]forskolin)、S5组(8.3 mmol/L葡萄糖溶液+10 nmol/L GLP-1+10 nmol/L ghrelin+10 μmol/L[10]6-Phe-cAMP);所有试剂均于前一试剂处理10 min后依次加入后一试剂共同处理。每10个胰岛放入1个1.5 mL离心管中,加入300 μL含相应浓度试剂的KRBH培养液(KRBH培养液购自美国Sigma公司;使用时添加1 mg/mL小牛血清白蛋白),于37 ℃、5% CO2条件下培养3 h。
1.5 ELISA法检测胰岛素及cAMP浓度吸取上清250 μL于新的离心管中,标记后4 ℃保存。按照大鼠胰岛素ELISA检测试剂盒操作说明处理样本,采用酶标分析仪测定450 nm波长下的光密度(D)值。同时,于原有离心管中加入0.5 mL KRBH培养液,超声波裂解胰岛。37 ℃ 400×g离心20 min后取上清400 μL,按照cAMP ELISA检测试剂盒操作说明处理样本后,用酶标仪测定450 nm波长下的D值。根据浓度和D值算出标准曲线的回归方程,分别采用三次样条回归模型和logistic曲线模型(四参数)计算胰岛素和cAMP浓度。
1.6 统计学处理应用SPSS 24.0软件进行数据分析。计量资料均以x±s表示,同组统计指标给予不同处理的变化比较采用单因素方差分析。检验水准(α)为0.05。
2 结果 2.1 DTZ及AO/PI染色结果分离、纯化后的胰岛被DTZ染成猩红色,镜下表现为大小不一的圆形或卵圆形细胞团和散在细胞(图 1A、1B)。胰岛细胞被AO/PI染色后,活细胞发出绿色荧光(图 1C、1D)。
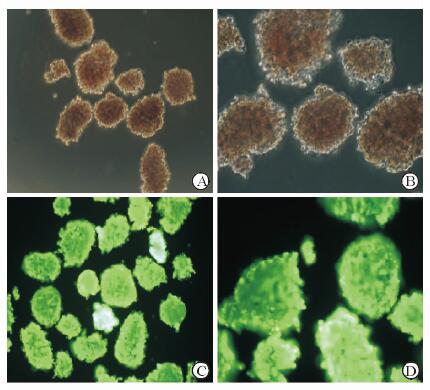
|
图 1 大鼠胰岛细胞DTZ及AO/PI染色 A, B:双硫腙(DTZ)染色; C, D:吖啶橙(AO)/碘化丙啶(PI)染色. Original magnification: ×100 (A,C), ×200 (B,D) |
2.2 Ghrelin对胰岛素和cAMP浓度的影响
由图 2可见,与对照组(S0组)相比,GLP-1(S1组)能够促进胰岛素分泌,并上调cAMP的浓度(P<0.05),而与ghrelin同时处理胰岛(S2组)可明显抑制GLP-1对胰岛素和cAMP的上调效应(P<0.05)。当GLP-1、ghrelin与GHSR-1α拮抗剂D-Lys3-GHRP-6联合作用时(S3组),结果显示胰岛素与cAMP浓度均明显增加,与GLP-1和ghrelin共同处理(S2组)相比差异有统计学意义(P<0.05);与forskolin(S4组)或6-Phe-cAMP(S5组)共同处理时,胰岛素和cAMP浓度也均高于S2组(P均<0.05)。
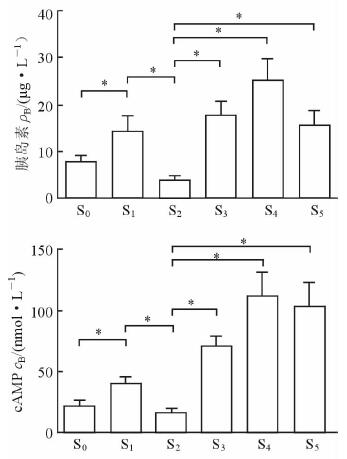
|
图 2 各组间胰岛细胞分泌胰岛素及释放cAMP比较 S0组: 8.3 mmol/L葡萄糖溶液(GS);S1组:8.3 mmol/L GS+10 nmol/L胰高血糖素样肽1(GLP-1);S2组:8.3 mmol/L GS+10 nmol/L GLP-1+10 nmol/L ghrelin;S3组:8.3 mmol/L GS+10 nmol/L GLP-1+10 nmol/L ghrelin+1 μmol/L生长激素促泌素受体1α(GHSR-1α)拮抗剂生长激素释放肽6(D-Lys3-GHRP-6);S4组:8.3 mmol/L GS+10 nmol/L GLP-1+10 nmol/L ghrelin+5 μmol/L腺苷酸环化酶激动剂毛喉素(forskolin);S5组:8.3 mmol/L GS+10 nmol/L GLP-1+10 nmol/L ghrelin+10 μmol/L PKA激动剂6-Phe-cAMP. cAMP:环磷酸腺苷. *P<0.05. n=5, x±s |
3 讨论
研究发现,摄食后GLP-1释放到循环中的浓度增加,禁食使得胰高血糖素原1和2表达均下降[11],还发现GLP-1的肠促胰素作用在2型糖尿病患者体内有所减弱[12];而循环ghrelin的浓度在餐前增加,在餐后下降[13],低血清ghrelin水平有助于改善机体糖代谢、胰岛素抵抗和肥胖,高血清ghrelin水平在胰岛中能够抑制胰岛素分泌,在中枢能显著增加摄食量[14]。可见GLP-1和ghrelin对于机体的糖代谢和胰岛素分泌存在一定的“相反性”。本研究也显示ghrelin可以拮抗GLP-1促胰岛素分泌的效应。
研究表明,cAMP/PKA通路在调控胰岛分泌胰岛素过程中发挥重要作用[9]。Ghrelin可通过抑制cAMP/PKA通路进而抑制胰岛素的分泌[15]。本研究结果显示,在8.3 mmol/L葡萄糖状态下,GLP-1促进了胰岛素的分泌和cAMP的释放,而ghrelin可以拮抗GLP-1的这种促进效应;但在给予ghrelin的主要结合受体GHSR-1α的拮抗剂D-Lys3-GHRP-6时,其胰岛素分泌和cAMP释放又明显增加。GLP-1、ghrelin共同处理胰岛释放cAMP的浓度明显低于其与forskolin或6-Phe-cAMP共同作用时的浓度。因此,我们得出结论:ghrelin能够抑制GLP-1的促胰岛素分泌效应,其作用机制可能是通过cAMP/PKA通路竞争性抑制GLP-1的促分泌效应。该结论为临床实践中治疗糖尿病提供了新的思路,即可以在阻断ghrelin的同时激活GLP-1,增加cAMP的释放,促进胰岛素分泌,从而达到治疗糖尿病的目的。
本研究仅在体外水平验证了ghrelin对GLP-1的促胰岛素分泌效应的拮抗作用,并探讨了与cAMP/PKA通路相关的可能机制,由于胰岛素分泌涉及的影响因素较多,排除其他因素对GLP-1和ghrelin的影响难度较大,故本研究并未开展体内实验。本研究虽只有体外实验结果,但可为后续体内研究提供科研思路,有助于进一步探究激素在体内外作用的差异。
| [1] | NARAYANASWAMI V, DWOSKIN L P. Obesity:current and potential pharmacotherapeutics and targets[J]. Pharmacol Ther, 2017, 170: 116–147. DOI: 10.1016/j.pharmthera.2016.10.015 |
| [2] | FAERCH K, HULMÁN A, SOLOMON T P. Heterogeneity of pre-diabetes and type 2 diabetes:implications for prediction, prevention and treatment responsiveness[J]. Curr Diabetes Rev, 2016, 12: 30–41. |
| [3] | SECHER A, JELSING J, BAQUERO A F, HECKSHER-SØRENSEN J, COWLEY M A, DALBØGE L S, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss[J]. J Clin Invest, 2014, 124: 4473–4488. DOI: 10.1172/JCI75276 |
| [4] | SALEHI M, GASTALDELLI A, D'ALESSIO D A. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass[J/OL]. Gastroenterology, 2014, 146:669-680. e2. doi:10.1053/j.gastro.2013.11.044. |
| [5] | LEE J H, NGUYEN Q N, LE Q A. Comparative effectiveness of 3 bariatric surgery procedures:Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy[J]. Surg Obes Relat Dis, 2016, 12: 997–1002. DOI: 10.1016/j.soard.2016.01.020 |
| [6] | MARTINUSSEN C, BOJSEN-MØLLER K N, DIRKSEN C, JACOBSEN S H, JØRGENSEN N B, KRISTIANSEN V B, et al. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass[J]. Am J Physiol Endocrinol Metab, 2015, 308: E535–E544. DOI: 10.1152/ajpendo.00506.2014 |
| [7] | GE G H, DOU H J, YANG S S, MA J W, CHENG W B, QIAO Z Y, et al. Glucagon-like peptide-1 protects against cardiac microvascular endothelial cells injured by high glucose[J]. Asian Pac J Trop Med, 2015, 8: 73–78. DOI: 10.1016/S1995-7645(14)60191-7 |
| [8] | CARTER J D, DULA S B, CORBIN K L, WU R, NUNEMAKER C S. A practical guide to rodent islet isolation and assessment[J]. Biol Proced Online, 2009, 11: 3–31. DOI: 10.1007/s12575-009-9021-0 |
| [9] | DAMDINDORJ B, DEZAKI K, KURASHINA T, SONE H, RITA R, KAKEI M, et al. Exogenous and endogenous ghrelin counteracts GLP-1 action to stimulate cAMP signaling and insulin secretion in islet β-cells[J]. FEBS Lett, 2012, 586: 2555–2562. DOI: 10.1016/j.febslet.2012.06.034 |
| [10] | KONG X, YAN D, WU X, GUAN Y, MA X. Glucotoxicity inhibits cAMP-protein kinase A-potentiated glucose-stimulated insulin secretion in pancreatic β-cells[J]. J Diabetes, 2015, 7: 378–385. DOI: 10.1111/jdb.2015.7.issue-3 |
| [11] | HOYT E C, LUND P K, WINESETT D E, FULLER C R, GHATEI M A, BLOOM S R, et al. Effects of fasting, refeeding, and intraluminal triglyceride on proglucagon expression in jejunum and ileum[J]. Diabetes, 1996, 45: 434–439. DOI: 10.2337/diab.45.4.434 |
| [12] | KJEMS L L, HOLST J J, VOLUND A, MADSBAD S. The influence of GLP-1 on glucose-stimulated insulin secretion:effects on β-cell sensitivity in type 2 and nondiabetic subjects[J]. Diabetes, 2003, 52: 380–386. DOI: 10.2337/diabetes.52.2.380 |
| [13] | CUMMINGS D E, PURNELL J Q, FRAYO R S, SCHMIDOVA K, WISSE B E, WEIGLE D S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans[J]. Diabetes, 2001, 50: 1714–1719. DOI: 10.2337/diabetes.50.8.1714 |
| [14] | YADA T, DEZAKI K, SONE H, KOIZUMI M, DAMDINDORJ B, NAKATA M, et al. Ghrelin regulates insulin release and glycemia:physiological role and therapeutic potential[J]. Curr Diabetes Rev, 2008, 4: 18–23. DOI: 10.2174/157339908783502352 |
| [15] | DEZAKI K, DAMDINDORJ B, SONE H, DYACHOK O, TENGHOLM A, GYLFE E, et al. Ghrelin attenuates cAMP-PKA signaling to evoke insulinostatic cascade in islet β-cells[J]. Diabetes, 2011, 60: 2315–2324. DOI: 10.2337/db11-0368 |
 2017, Vol. 38
2017, Vol. 38


