2. 解放军161医院急诊科, 武汉 430000
2. Department of Emergency, No. 161 Hospital of PLA, Wuhan 430000, Hubei, China
缺血缺氧性脑病(hypoxic-ischemic encephalopathy,HIE)是由多种原因引起的局部或全脑血供不足而导致的缺血缺氧性脑损伤,常见于脑梗死、心跳骤停、卒中、窒息、溺水等疾病,表现为不同程度的神经功能障碍,如失语、偏瘫、认知能力下降、植物人状态,严重者甚至会死亡[1]。在常规治疗之外,亚低温治疗、高压氧治疗、药物治疗等方法相继应用在HIE的治疗中,取得了一些进展;但由于产生了不可逆的脑损伤,患者的出院率及生活质量仍然不高[2]。近年来,骨髓间充质干细胞(bone marrow mesenchymal stem cell,BMSC)在治疗HIE的研究中取得了良好效果,但也存在提取率低、取材过程痛苦、分化能力下降等不足[3]。脂肪间充质干细胞(adipose-derived mesenchymal stem cell,ADMSC)不仅具有与BMSC相似的生物学特性及多向分化潜能[4],而且具有脂肪组织来源丰富、提取率高、取材过程痛苦程度低等特点[5],使其具有替代BMSC成为再生医学新的干细胞来源的潜能。本文对ADMSC在HIE治疗中的应用进行综述。
1 ADMSC的生物学特性ADMSC广泛存在于人体脂肪中,不仅取材方便,而且每1 g脂肪组织中可获得(0.5~2)×106个血管基质成分(stromal vascular fraction,SVF)细胞,SVF细胞经过培养分离可获得1%的ADMSC(0.5×104~2×105),其比例远高于骨髓分离。在免疫表型方面,与其他来源的间充质干细胞(mesenchymal stem cell,MSC)类似,ADMSC表达MSC特异性表面抗原,如CD90、CD105、CD73,不表达造血干细胞表面抗原,如CD45、CD34,不表达人类白细胞DR抗原(HLA-DR)[6-7]。同时,ADMSC具有多向分化能力,能分化为骨、软骨、脂肪,并且分化、增殖能力稳定,且不会随供者年龄增长出现明显下降趋势[3, 8]。体外实验证实,ADMSC经多次传代后仍具有稳定的增殖能力,其增殖能力优于BMSC;ADMSC分泌的吲哚胺2, 3二加氧酶活性水平明显优于BMSC,具有更强的免疫调节能力[9]。
2 ADMSC治疗HIE的相关机制 2.1 归巢机制归巢是指MSC在多种因素作用下,定向性迁移越过血管内皮细胞到达靶向组织,并定植存活的过程。在大鼠大脑中动脉闭塞模型(middle cerebral artery occlusion,MCAO)中,通过颈内动脉或外周静脉注射ADMSC,发现ADMSC能穿越血脑屏障迁移至脑损伤区域[10-11],其机制可能与基质细胞衍生因子1α(stromal cell-derived factor 1α,SDF-1α)和趋化因子受体4(C-X-C chemokine receptor type 4,CXCR4) 轴有关。SDF-1α是促进ADMSC迁移的关键因子,损伤部位SDF-1α表达增加或者ADMSC过表达SDF-1α都能促进ADMSC向损伤部位的迁移[12],而CXCR4抑制剂AMD3100能抑制迁移能力[13]。在细胞信号通路方面,激活丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)通路、PI3K/Akt通路、Ras同源基因家族、血小板衍生生长因子BB/血小板衍生生长因子受体β通路都能加强ADMSC的迁移能力,促进归巢[14]。
2.2 旁分泌功能ADMSC能分泌多种营养因子,这些因子多为水溶性物质,能在脑损伤部位发挥保护及修复作用。其中,脑源性神经生长因子(brain-derived neurotrophic factor,BDNF)和神经生长因子(nerve growth factor,NGF)具有促进新的神经元分化及强大的保护受损神经元的作用[15-16];血管内皮生长因子(vascular endothelial growth factor,VEGF)和肝细胞生长因子(hepatocyte growth factor,HGF)是促进血管再生的关键因子,能促进损伤部位的血管再生,为损伤部位恢复血液供应[17-18]。研究发现ADMSC上清液含有多种因子,在大鼠HIE模型中注射上清液,发现神经细胞凋亡减少,胶质细胞增殖减轻,新生血管形成,脑功能改善[19]。不同来源的MSC分泌的因子存在差异,ADMSC分泌某些蛋白(成纤维细胞生长因子、干扰素γ等)的能力优于BMSC[9]。
2.3 免疫调节在脑损伤后数小时内,就会出现大量的黏附于受损部位的内皮细胞神经炎症介质,这些介质能加重已存在的神经损伤或者在神经修复中起积极作用。体外实验证实,ADMSC能抑制单核细胞增殖,并调节白介素(IL)6、转化生长因子1的浓度[20]。动物实验表明,ADMSC既能下调促炎因子(IL-18、TNF-α、Toll样受体4等),也能上调抗炎因子(IL-8、Bcl-2蛋白等),从而减轻炎症反应[10, 21]。其潜在作用机制目前尚不明确,可能是ADMSC通过与细胞的直接接触及分泌可溶性因子共同作用而发挥一系列效应。
2.4 神经样分化ADMSC除了能向中胚层组织分化,还具有跨胚层向神经细胞分化的能力,即横向分化能力,其分化机制可能与细胞所处的微环境有关。Safford等[22]发现,在一定环境下ADMSC(传代4~5代)可分化成类神经元样细胞,并表达神经巢蛋白、核蛋白等神经表型,在开始诱导后1~3 h,ADMSC就可以出现阳性神经表型。体外实验发现,多种因素及条件(富含血小板的血清[23]、人参皂苷Rg1[24]、神经营养因子[25]、催产素[26])均可诱导AMDSC向神经样细胞分化。在动物实验中,无论进行颅内定位或外周血管注射都会在脑损伤区域发现ADMSC,并表达巢蛋白、核蛋白等神经表型[11, 27],说明ADMSC产生了神经样分化。上述研究表明,多种物质均能诱导ADMSC向神经样细胞分化,而这些物质可能通过激活或失活某些细胞内与细胞增殖分化相关的信号通路来诱导神经样分化[28],激活MAPK/ERK通路、Wnt通路并促进ADMSC神经样分化[28-29]。
2.5 内源性神经再生成人大脑的侧脑室室下区(subventricular zone,SVZ)及海马齿状回颗粒下层(subgranular zone,SGZ)的神经细胞仍保持着一定的神经再生能力,在HIE发生2周内SVZ的神经干细胞增殖现象明显[30],提示如果能够大量激活SVZ、SGZ的神经干细胞,就可以通过加强内源性神经再生促进神经修复。注射ADMSC 1周后,SVZ神经干细胞增殖、分化增多,出现了大量BrdU、DCX双阳性细胞,并且向脑损伤区域迁移,说明ADMSC能促使SVZ的神经干细胞向神经细胞分化[10, 31]。BDNF能调控中枢神经细胞存活、增殖,以及神经分化[32],ADMSC促进内源性神经再生可能与其大量分泌BDNF、NGF多种营养因子有关[33]。
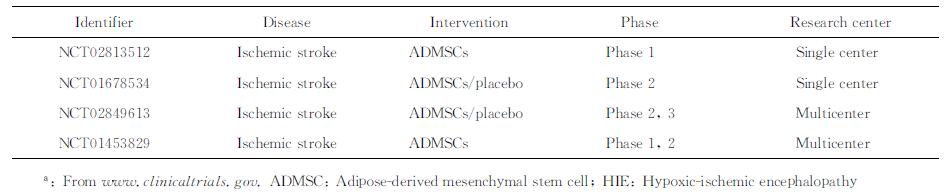
3 ADMSC治疗HIE的临床研究 3.1 ADMSC的临床试验2016年12月16日从www.clinicaltrials.gov查询到使用ADMSC治疗HIE的临床试验共4项,见表 1。已开展的试验侧重于对ADMSC治疗缺血性卒中的有效性及安全性评价,研究对象均为60~80岁脑梗死患者,注射剂量为1×106细胞/kg,注射方式为静脉注射或颈内动脉注射,注射时间为卒中后1~14 d,观察时间6~24个月。Regenerative Stem Cell Therapy for Stroke in Europe(NCT02849613) 试验是2016年在欧洲开展的多中心、大样本(400例)临床研究,研究对象为18岁以上的缺血性卒中患者,设置安慰剂对照组,观察静脉注射ADMSC治疗缺血性卒中的有效性和安全性,预计在2020年9月完成。该试验缺乏对心搏骤停、窒息、失血等原因引起的全脑缺血的研究,因此需要进一步开展针对全脑缺血的大样本、多中心临床试验,验证ADMSC在治疗HIE中的有效性和安全性。
|
|
表 1 应用ADMSC治疗HIE的临床试验a Tab 1 Clinical trials of HIE treated with ADMSCsa |
3.2 ADMSC体内应用的安全性
ADMSC不表达HLA-DR,不会引起效应T细胞介导的免疫排斥反应,因此目前无论在异体还是异种ADMSC移植实验中,均未发现存在排异反应。ADMSC具有归巢、多向分化、分泌营养因子的特点,因此致瘤性是其安全性研究的焦点。ADMSC是否会定向迁移至病灶,分泌生长因子,促进肿瘤细胞增殖、浸润及远处转移,是否会诱发新生肿瘤,目前尚存争议。Feng等[34]发现,ADMSC会向胶质细胞瘤迁移,但在体外不会分化为肿瘤相关的成纤维细胞,在体内也不具有致瘤性。但也有研究认为,ADMSC通过分泌IL-6激活肿瘤细胞内的JAK2/STAT3信号通路,可能促进乳腺肿瘤、结肠癌发生[35],ADMSC能促进与肥胖相关肿瘤(如子宫内膜癌)的生长[36-37]。目前关于ADMSC安全性的研究较少,缺乏长时间的观察及相关机制的探讨。
3.3 ADMSC临床应用的瓶颈 3.3.1 供体选择ADMSC移植包括自体移植和异体移植。自体移植是指从患者体内抽取脂肪,需要传代3~6次才能获得临床治疗需要的细胞,准备时间长达4周。ADMSC低表达MHC Ⅰ类抗原,不表达MHC Ⅱ类抗原和共刺激分子CD40、CD80y、CD86,具有免疫调节和免疫豁免的特性,此特点使异体甚至异种移植成为可能。研究发现,人来源的ADMSC对大鼠HIE具有较好的治疗效果[38],且与同种来源ADMSC治疗效果无明显差异[17],为临床异体移植提供了理论支持。从健康供者抽取脂肪进行培养和传代,准备时间可缩短至7 d。此外,脂肪的类型、脂肪采集的部位、采集的方法、供者的体质量、健康状态等多种因素都可能影响ADMSC的质量,因此需要对供者进行筛选,对采集过程进行规范。
3.3.2 注射途径注射途径主要有颅内定位注射、鞘内注射和静脉注射3种。颅内定位注射ADMSC的归巢细胞数多,但存在不能大剂量注射、易引起继发性损伤等缺点[39]。鞘内注射相对风险较小,但存在操作烦琐、容易导致脊髓损伤等不足。静脉注射安全、快捷,可大剂量注射,但存在ADMSC归巢数量少、部分细胞被肺毛细血管网捕获的缺点[39]。临床上,需要结合患者的特点,选择合适的注射途径。
3.3.3 治疗时间窗研究发现,在HIE发生后30 min注射ADMSC,24 h内就能减轻神经损伤,且在观察期(14 d)内也一直有脑保护作用[40]。也有研究表明,在脑损伤后30 d注射BMSC,没有观察到症状好转[41]。上述差异表明,早期注射能通过多个环节保护脑神经,但有效的时间窗需要新的研究来确认。
4 小结ADMSC能够通过归巢、旁分泌、免疫调节、神经样分化、内源性神经再生等多个环节减轻HIE的脑损伤,在动物实验中取得了较好的效果,并开展了初步的临床试验。但从临床应用角度而言,目前仍存在一些问题有待解决,如最佳注射途径、治疗的时间窗、作用机制、与肿瘤关系等。随着研究的不断深入,ADMSC有望成为治疗HIE的理想策略。
| [1] | EID S M, ABOUGERGI M S, ALBAENI A, CHANDRA-STROBOS N. Survival, expenditure and disposition in patients following out-of-hospital cardiac arrest:1995-2013[J]. Resuscitation, 2017, 113: 13–20. DOI: 10.1016/j.resuscitation.2016.12.027 |
| [2] | ROCHA-FERREIRA E, HRISTOVA M. Plasticity in the neonatal brain following hypoxic-ischaemic injury[J]. Neural Plast, 2016, 2016: 4901014. |
| [3] | BEANE O S, FONSECA V C, COOPER L L, KOREN G, DARLING E M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells[J/OL]. PLoS One, 2014, 26:e115963. doi:10.1371/journal.pone.0115963. |
| [4] | WOO D H, HWANG H S, SHIM J H. Comparison of adult stem cells derived from multiple stem cell niches[J]. Biotechnol Lett, 2016, 38: 751–759. DOI: 10.1007/s10529-016-2050-2 |
| [5] | FRESE L, DIJKMAN P E, HOERSTRUP S P. Adipose tissue-derived stem cells in regenerative medicine[J]. Transfus Med Hemother, 2016, 43: 268–274. DOI: 10.1159/000448180 |
| [6] | BALOLONG E, LEE S, NEMENO J G, LEE J I. Are they really stem cells? Scrutinizing the identity of cells and the quality of reporting in the use of adipose tissue-derived stem cells[J]. Stem Cells Int, 2016, 2016: 2302430. |
| [7] | BOURIN P, BUNNELL B A, CASTEILLA L, DOMINICI M, KATZ A J, MARCH K L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells:a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT)[J]. Cytotherapy, 2013, 15: 641–648. DOI: 10.1016/j.jcyt.2013.02.006 |
| [8] | SHAN X, ROBERTS C, KIM E J, BRENNER A, GRANT G, PERCEC I. Transcriptional and cell cycle alterations mark aging of primary human adipose-derived stem cells[J]. Stem Cells, 2017, 35: 1392–1401. DOI: 10.1002/stem.2592 |
| [9] | LI C Y, WU X Y, TONG J B, YANG X X, ZHAO J L, ZHENG Q F, et al. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy[J]. Stem Cell Res Ther, 2015, 6: 55. DOI: 10.1186/s13287-015-0066-5 |
| [10] | OH S H, CHOI C, CHANG D J, SHIN D A, LEE N, JEON I, et al. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells[J]. Cytotherapy, 2015, 17: 1090–1103. DOI: 10.1016/j.jcyt.2015.04.007 |
| [11] | JIANG W, LIANG G, LI X, LI Z, GAO X, FENG S, et al. Intracarotid transplantation of autologous adipose-derived mesenchymal stem cells significantly improves neurological deficits in rats after MCAo[J]. J Mater Sci Mater Med, 2014, 25: 1357–1366. DOI: 10.1007/s10856-014-5157-9 |
| [12] | CAI A, QIU R, LI L, ZHENG D, DONG Y, YU D, et al. Atorvastatin treatment of rats with ischemia-reperfusion injury improves adipose-derived mesenchymal stem cell migration and survival via the SDF-1α/CXCR-4 axis[J/OL]. PLoS One, 2013, 8:e79100. doi:10.1371/journal.pone.0079100. |
| [13] | YU Q, LIU L, LIN J, WANG Y, XUAN X, GUO Y, et al. SDF-1α/CXCR4 axis mediates the migration of mesenchymal stem cells to the hypoxic-ischemic brain lesion in a rat model[J]. Cell J, 2015, 16: 440–447. |
| [14] | ZHAO Y, ZHANG H. Update on the mechanisms of homing of adipose tissue-derived stem cells[J]. Cytotherapy, 2016, 18: 816–827. DOI: 10.1016/j.jcyt.2016.04.008 |
| [15] | LI X, ZHENG W, BAI H, WANG J, WEI R, WEN H, et al. Intravenous administration of adipose tissue-derived stem cells enhances nerve healing and promotes BDNF expression via the TrkB signaling in a rat stroke model[J]. Neuropsychiatr Dis Treat, 2016, 12: 1287–1293. |
| [16] | HAN C, SONG L, LIU Y, ZOU W, JIANG C, LIU J. Rat cortex and hippocampus-derived soluble factors for the induction of adipose-derived mesenchymal stem cells into neuron-like cells[J]. Cell Biol Int, 2014, 38: 768–776. DOI: 10.1002/cbin.v38.6 |
| [17] | GUTIERREZ-FERNANDEZ M, RODRIGUEZ-FRUTOS B, RAMOS-CEJUDO J, OTERO-ORTEGA L, FUENTES B, VALLEJO-CREMADES M T, et al. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct:proof of concept in rats[J]. J Transl Med, 2015, 13: 46. DOI: 10.1186/s12967-015-0406-3 |
| [18] | RIBEIRO C A, FRAGA J S, GRAOS M, NEVES N M, REIS R L, GIMBLE J M, et al. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations[J]. Stem Cell Res Ther, 2012, 3: 18. DOI: 10.1186/scrt109 |
| [19] | CHO Y J, SONG H S, BHANG S, LEE S, KANG B G, LEE J C, et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke[J]. J Neurosci Res, 2012, 90: 1794–1802. DOI: 10.1002/jnr.v90.9 |
| [20] | MELIEF S M, ZWAGINGA J J, FIBBE W E, ROELOFS H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts[J]. Stem Cells Transl Med, 2013, 2: 455–463. DOI: 10.5966/sctm.2012-0184 |
| [21] | LI D, FANG Y, WANG P, SHAN W, ZUO Z, XIE L. Autologous transplantation of adipose-derived mesenchymal stem cells attenuates cerebral ischemia and reperfusion injury through suppressing apoptosis and inducible nitric oxide synthase[J]. Int J Mol Med, 2012, 29: 848–854. |
| [22] | SAFFORD K M, HICOK K C, SAFFORD S D, HALVORSEN Y D, WILKISON W O, GIMBLE J M, et al. Neurogenic differentiation of murine and human adipose-derived stromal cells[J]. Biochem Biophys Res Commun, 2002, 294: 371–379. DOI: 10.1016/S0006-291X(02)00469-2 |
| [23] | LI H, HAN Z, LIU D, ZHAO P, LIANG S, XU K. Autologous platelet-rich plasma promotes neurogenic differentiation of human adipose-derived stem cells in vitro[J]. Int J Neurosci, 2013, 123: 184–190. DOI: 10.3109/00207454.2012.742077 |
| [24] | XU F T, LI H M, YIN Q S, CUI S E, LIU D L, NAN H, et al. Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro[J]. Can J Physiol Pharmacol, 2014, 92: 467–475. DOI: 10.1139/cjpp-2013-0377 |
| [25] | RAZAVI S, RAZAVI M R, KHEIROLLAHI-KOUHESTANI M, MARDANI M, MOSTAFAVI F S. Co-culture with neurotrophic factor secreting cells induced from adipose-derived stem cells:promotes neurogenic differentiation[J]. Biochem Biophys Res Commun, 2013, 440: 381–387. DOI: 10.1016/j.bbrc.2013.09.069 |
| [26] | JAFARZADEH N, JAVERI A, KHALEGHI M, TAHA M F. Oxytocin improves proliferation and neural differentiation of adipose tissue-derived stem cells[J]. Neurosci Lett, 2014, 564: 105–110. DOI: 10.1016/j.neulet.2014.02.012 |
| [27] | YANG Y C, LIU B S, SHEN C C, LIN C H, CHIAO M T, CHENG H C. Transplantation of adipose tissue-derived stem cells for treatment of focal cerebral ischemia[J]. Curr Neurovasc Res, 2011, 8: 1–13. DOI: 10.2174/156720211794520215 |
| [28] | SALEHI H, AMIRPOUR N, NIAPOUR A, RAZAVI S. An overview of neural differentiation potential of human adipose derived stem cells[J]. Stem Cell Rev, 2016, 12: 26–41. DOI: 10.1007/s12015-015-9631-7 |
| [29] | HU F, SUN B, XU P, ZHU Y, MENG X H, TENG G J, et al. MiR-218 induces neuronal differentiation of ASCs in a temporally sequential manner with fibroblast growth factor by regulation of the Wnt signaling pathway[J]. Sci Rep, 2017, 7: 39427. DOI: 10.1038/srep39427 |
| [30] | ARVIDSSON A, COLLIN T, KIRIK D, KOKAIA Z, LINDVALL O. Neuronal replacement from endogenous precursors in the adult brain after stroke[J]. Nat Med, 2002, 8: 963–970. DOI: 10.1038/nm747 |
| [31] | SCHWERK A, ALTSCHULER J, ROCH M, GOSSEN M, WINTER C, BERG J, et al. Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning[J]. Cytotherapy, 2015, 17: 199–214. DOI: 10.1016/j.jcyt.2014.09.005 |
| [32] | BAO X, WEI J, FENG M, LU S, LI G, DOU W, et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats[J]. Brain Res, 2011, 1367: 103–113. DOI: 10.1016/j.brainres.2010.10.063 |
| [33] | JEONG C H, KIM S M, LIM J Y, RYU C H, JUN J A, JEUN S S. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model[J]. Biomed Res Int, 2014, 2014: 129145. |
| [34] | FENG Y, ZHU M, DANGELMAJER S, LEE Y M, WIJESEKERA O, CASTELLANOS C X, et al. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer[J/OL]. Cell Death Dis, 2014, 5:e1567. doi:10.1038/cddis.2014.521. |
| [35] | WEI H J, ZENG R, LU J H, LAI W F, CHEN W H, LIU H Y, et al. Adipose-derived stem cells promote tumor initiation and accelerate tumor growth by interleukin-6 production[J]. Oncotarget, 2015, 6: 7713–7726. DOI: 10.18632/oncotarget |
| [36] | STRONG A L, BUROW M E, GIMBLE J M, BUNNELL B A. Concise review:the obesity cancer paradigm:exploration of the interactions and crosstalk with adipose stem cells[J]. Stem Cells, 2015, 33: 318–326. DOI: 10.1002/stem.1857 |
| [37] | FREESE K E, KOKAI L, EDWARDS R P, PHILIPS B J, SHEIKH M A, KELLEY J, et al. Adipose-derived stems cells and their role in human cancer development, growth, progression, and metastasis:a systematic review[J]. Cancer Res, 2015, 75: 1161–1168. DOI: 10.1158/0008-5472.CAN-14-2744 |
| [38] | CHEN K H, CHEN C H, WALLACE C G, YUEN C M, KAO G S, CHEN Y L, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke[J]. Oncotarget, 2016, 7: 74537–74556. |
| [39] | DU G, LIU Y, DANG M, ZHU G, SU R, FAN Y, et al. Comparison of administration routes for adipose-derived stem cells in the treatment of middle cerebral artery occlusion in rats[J]. Acta Histochemica, 2014, 116: 1075–1084. DOI: 10.1016/j.acthis.2014.05.002 |
| [40] | GUTIÉRREZ-FERNÁNDEZ M, RODRÍGUEZ-FRUTOS B, RAMOS-CEJUDO J, OTERO-ORTEGA L, FUENTES B, VALLEJO-CREMADES M T, et al. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct:proof of concept in rats[J]. J Transl Med, 2015, 13: 46. DOI: 10.1186/s12967-015-0406-3 |
| [41] | DE VASCONCELOS D S A, DA C R J, DIAZ P B, MORAES L, JASMIN J, GIRALDI-GUIMARAES A, et al. Therapeutic window for treatment of cortical ischemia with bone marrow-derived cells in rats[J]. Brain Res, 2010, 1306: 149–158. DOI: 10.1016/j.brainres.2009.09.094 |
 2017, Vol. 38
2017, Vol. 38