恶性黑素瘤是临床上一种高度恶性的肿瘤,其发病率在全球男性及女性中分别排第5和第6位,病死率高达23.2%[1]。恶性黑素瘤常原发于皮肤及黏膜组织,早期症状不明显,生长速度快,通常伴随局部侵犯,也可广泛侵犯表皮、皮下组织以及深层结缔组织,甚至相邻器官(或组织),也可通过淋巴结、血行远处转移或广泛播散。黑素瘤的疗效和预后与转移灶数量密切相关,而临床上许多患者在确诊时已经属于中晚期,伴随多处转移,导致预后不良[2]。上皮间质转化(epithelial-mesenchymal transition,EMT)指上皮细胞在特定生理或病理情况下向间质细胞表型转变的过程。EMT与肿瘤的发生、发展尤其是侵袭转移密切相关[3]。EMT的现象也存在于黑素瘤中[4],因此,揭示EMT的分子调控机制及其与黑素瘤侵袭转移的关系,对于预防和治疗黑素瘤具有重要意义。
微小RNA(microRNA,miRNA)是一类长度不超过25 nt的内源性非编码RNA。MiRNA是近年来生命科研的明星分子,目前在人体已发现超过1 000种miRNA,它们广泛参与到机体的多种生理和病理过程,如细胞增殖、分化凋亡、迁移侵袭、器官发育、肿瘤发生和发展等[5]。MiRNA主要作用于编码基因的转录后水平,可以抑制靶基因的表达,在肿瘤发生、发展中miRNA被归纳为抑癌基因和癌基因两类。现在已知黑素瘤的发生、发展经历一个多步骤的复杂过程,涉及许多癌基因、抑癌基因以及信号转导的异常激活或失活。研究显示多种miRNA参与调控黑素瘤细胞的生物学行为,如miR-330-5p、miR-542-3p等可以抑制黑素瘤细胞迁移和侵袭[6-7]。
MicroRNA-205(miR-205) 是一种具有抑癌基因特征的miRNA,在多种肿瘤中表达降低,恢复其表达可以抑制多种肿瘤细胞增殖,如黑素瘤[8]。临床标本研究显示,miR-205在黑素瘤组织中表达减少,其表达水平与黑素瘤的分期呈正相关,与患者预后呈负相关[1]。以上研究提示miR-205可能调控黑素瘤细胞的迁移和侵袭,但是目前关于miR-205与黑素瘤细胞EMT关系以及其调控的分子机制尚不明了。因此,本研究通过miR-205过表达以探讨miR-205对黑素瘤细胞EMT、迁移和侵袭的作用及机制。
1 材料和方法 1.1 细胞培养人黑素瘤细胞株A375和A2058购自中国科学院上海分院细胞库,均采用含10%胎牛血清(FBS)和1%青-链霉素混合液的DMEM培养液培养,培养条件为5% CO2、37 ℃的细胞培养箱,生长方式为贴壁生长。每天换液,当细胞融合度达80%~90%时,采用0.25%胰蛋白酶消化传代,继续培养,用第4或第5代细胞进行后续实验。
1.2 MiR-205过表达慢病毒的转染MiR-205过表达慢病毒(Lenti-miR-205) 和空白对照慢病毒购自上海基科生物化学有限公司[对照病毒和miR205过表达病毒均携带绿色荧光蛋白(GFP)],分别称为miR-205组和NC组。慢病毒转染步骤如下:取生长良好的A375和A2058细胞接种于12孔板培养24 h,加入含有慢病毒浓度为107 U/mL的完全培养液孵育24 h,然后去掉含有慢病毒的培养液,换成1 mL新鲜培养液继续培养过夜,然后将细胞1:3传代,并继续培养1~2 d。为了获得稳转细胞株,采用含2 μg/mL嘌呤霉素的培养液进行培养,每3 d更换培养液,2周后获得稳转细胞株。
1.3 划痕实验检测细胞迁移能力在6孔板分别培养A375和A2058细胞(5×105/孔),贴壁过夜后用枪头垂直比着直尺在培养皿底部划线,注意角度不要倾斜。用PBS洗细胞3次,洗掉划下的细胞,用含2% FBS的培养液在37 ℃、5% CO2培养箱内培养,分别在0、8、24 h时随机选取3个不同视野下拍照,与0 h进行比较并计算迁移宽度。计算公式:拍照观察时相对迁移宽度=拍照观察时迁移宽度/0 h时平均划痕宽度。
1.4 Transwell实验检测细胞的侵袭能力在24孔板Transwell小室上室底部中央垂直加入100 μL稀释后的基质胶(1 mg/mL;用4 ℃预冷的无FBS培养液配制),37 ℃温育4~5 h使其呈胶状。细胞FBS饥饿2 h后,取200 μL细胞密度为2.5×104/mL的细胞悬液加入Transwell小室上室,贴壁过夜后换无FBS培养液。Transwell小室下室加入500 μL含20%FBS的培养液培养24 h后,用4%多聚甲醛室温固定30 min、0.1%结晶紫染色30 min、棉签拭去上室内的细胞,电子显微镜观察并计数紧贴上室底部膜下的细胞数。
1.5 激光共聚焦扫描显微镜观察细胞形态与蛋白表达在96孔板中接种约5×103/孔的细胞,贴壁培养24 h后用4%多聚甲醛4 ℃固定15 min,PBS清洗3次;再用体积分数为0.2%的Triton-Ⅹ 100破膜15 min,PBS清洗3次;然后用羊血清封闭1.5 h,PBS清洗3次,4 ℃孵育过夜标记β-actin(1:100;60008-1-lg,Proteintech公司),PBS清洗3次,连接DyLight 594的二抗(1:200) 处理1 h,DAPI复染5 min,PBS清洗3次,用防荧光淬灭封片剂封片。采用激光扫描共聚焦显微镜观察目的蛋白的荧光表达情况:DyLight 594激发波长594 nm,发射波长618 nm;DAPI激发波长358 nm,发射波长461 nm。
1.6 蛋白质印迹法检测EMT相关蛋白的表达离心收集细胞,RIPA裂解液处理30 min后,4 ℃ 10 000×g离心10 min,分离上清。蛋白经变性后,加入SDS-PAGE凝胶的上样孔,电泳、转膜(NC膜)、10%脱脂奶粉封闭1 h;分别于4 ℃过夜标记特异性一抗E-cadherin(3195)、波形蛋白(vimentin,3932)、Zeb1(3396)、整合素(intergrin,3629;均购自Cell Signaling公司)和Twist(ab50887)、基质金属蛋白酶(MMP)-2(2763-1)、MMP-9(2551-1;均购自Abcam公司),所有目的抗体稀释比例均为1:1 000。以GAPDH作为内参照蛋白(稀释比例为1:5 000;60004-1-lg,Proteintech公司)。TBST洗膜3次5 min;标记相应二抗(1:1 000稀释),孵育1 h,TBST洗3次5 min;暗室中膜上滴加适量ECL发光液,采用Tanon-6100全自动化学发光图像分析系统检测发光强度。使用Quantity One 4.31软件定量各条带的灰度值,根据公式计算目的蛋白的相对灰度值:目的蛋白的相对灰度值=目的蛋白的灰度值/GAPDH灰度值。
1.7 qPCR检测miR-205 mRNA的表达采用TRIzol法提取细胞总RNA,用赛默飞Nanodrop 2000/2000C分光光度计测定总RNA浓度。然后用反转录试剂盒(广州市锐博生物科技有限公司)反转录获得cDNA。MiRNA引物使用Bulge-LoopTM miRNA qPCR Primer Set(广州市锐博生物科技有限公司);按qPCR试剂盒(广州市锐博生物科技有限公司)操作说明进行qPCR反应。qPCR反应条件:95 ℃ 30 s;93 ℃ 5 s、60 ℃ 30 s、95 ℃ 15 s,35个循环;60 ℃ 1 min;95 ℃ 15 s。
1.8 统计学处理应用SPSS 19.0软件进行数据分析。计量资料用x±s表示,两组间比较采用Student’s t检验。所有实验重复3次。检验水准(α)为0.05。
2 结果 2.1 Lenti-miR-205稳转A375和A2058细胞株构建成功慢病毒(Lenti-miR-205) 对A375(图 1A)和A2058(图 1B)细胞的转染效率较高。qPCR结果显示,miR-205组两种细胞的miR-205 mRNA表达水平高于NC组(A375:251.00±57.90 vs 1.00±0.08,P < 0.01;A2058:52.90±4.80 vs 1.00±0.02,P < 0.01),提示miR-205过表达的A375和A2058稳转株构建成功。
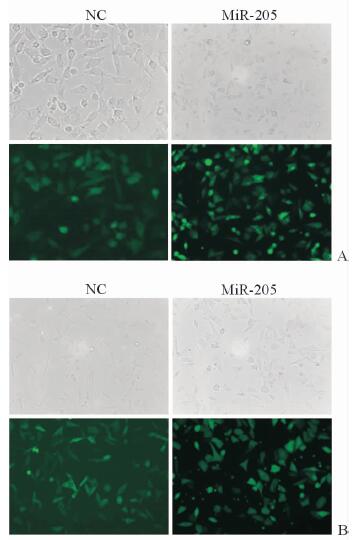
|
图 1 MiR-205过表达稳转A375和A2058细胞株的构建 Fig 1 Establishment of A375 and A2058 cells over-expressing miR-205 A: A375 cells; B: A2058 cells. NC group: The A375 and A2058 cells transfected with blank vector virus; miR-205 group: The A375 and A2058 cells transfected with Lenti-miR-205. Original magnification: ×200 |
2.2 MiR-205过表达抑制A375和A2058细胞的迁移能力
如图 2所示,划痕后细胞培养至8 h时,miR-205组A375和A2058细胞的相对划痕宽度均宽于NC组(P < 0.01);24 h时NC组划痕处均长满细胞,而miR-205组痕宽依然存在,两组差异有统计学意义(P < 0.01)。

|
图 2 划痕实验检测miR-205过表达对A375和A2058细胞迁移能力的影响 Fig 2 Effect of miR-205 over-expression on migration ability of A375 and A2058 cells by scratch assay A, C: A375 cells; B, D: A2058 cells. NC group: The A375 and A2058 cells transfected with blank vector virus; miR-205 group: The A375 and A2058 cells transfected with Lenti-miR-205. 0 h, 8 h and 24 h indicate that the cells cultured for 0, 8 and 24 h after scratching. Original magnification: ×100 (A, B).**P < 0.01 vs NC group. n=3, x±s |
2.3 MiR-205过表达抑制A375和A2058细胞的侵袭能力
Transwell实验结果(图 3)显示,miR-205组A375和A2058细胞的穿膜数量均少于NC组(P < 0.01),提示miR-205过表达能够减弱A375和A2058细胞的侵袭能力。

|
图 3 Transwell实验检测miR-205过表达对A375和A2058细胞侵袭能力的影响 Fig 3 Effect of miR-205 over-expression on invasion ability of A375 and A2058 cells by Transwell assay NC group: The A375 and A2058 cells transfected with blank vector virus; miR-205 group: The A375 and A2058 cells transfected with Lenti-miR-205. Original magnification:×100. **P < 0.01 vs NC group. n=3, x±s |
2.4 MiR-205过表达对A375和A2058细胞形态的影响
由图 4可见,NC组A375和A2058细胞均多为梭型或长条型,而miR-205组两种细胞均变为圆形、短杆型,提示miR-205过表达后A375和A2058细胞的形态特征由基质型向表皮型转化。

|
图 4 激光共聚焦扫描显微镜观察miR-205过表达对A375和A2058细胞形态的影响 Fig 4 Effect of miR-205 over-expression on morphology of A375 and A2058 cells by confocal laser scanning microscopy NC group: The A375 and A2058 cells transfected with blank vector virus; miR-205 group: The A375 and A2058 cells transfected with Lenti-miR-205. Original magnification:×400 |
2.5 MiR-205过表达对A375和A2058细胞中EMT相关蛋白表达的影响
MiR-205组A375和A2058细胞内E-cadherin蛋白表达均高于NC组(P < 0.01),而vimentin、intergrin、Twist、Zeb1、MMP-2和MMP-9蛋白的表达均低于NC组(P < 0.01,见图 5A~5C。激光共聚焦扫描显微镜结果显示,miR-205组两种细胞内E-cadherin表达数量与荧光强度较NC组增加(图 5D)。
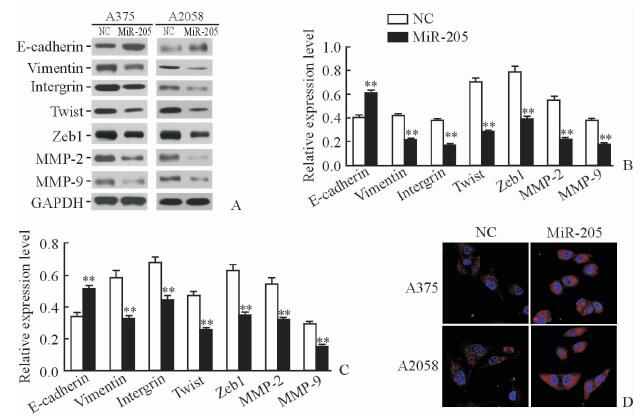
|
图 5 MiR-205过表达对EMT相关蛋白表达水平的影响 Fig 5 Effect of miR-205 over-expression on expressions of EMT-related proteins A-C: The expressions of EMT-related proteins were detected by Western blotting (B: A375 cells; C: A2058 cells); D: The expression of E-cadherin was detected by confocal laser scanning microscopy. NC group: The A375 and A2058 cells transfected with blank vector virus; miR-205 group: The A375 and A2058 cells transfected with Lenti-miR-205. EMT: Epithelial-mesenchymal transition; MMP: Matrix metalloproteinase; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase. **P < 0.01 vs NC group. n=3, x±s. Original magnification:×400 (D) |
3 讨论
黑素瘤发源于表皮基底层的黑色素细胞,细胞具有极性,细胞间排列紧密,具有非常广泛的紧密连接。众多的细胞分子在维持肿瘤细胞形态和细胞间连接中发挥重要作用,如E-cadherin等[9]。黑素瘤细胞是如何打破这种连接方式,从原发部位游离,进而启动肿瘤的侵袭和转移,并如何重塑其生存的微环境,其具体机制尚不清楚。近年来,EMT在肿瘤侵袭和转移中的作用逐渐成为人们关注的热点,被认为是肿瘤发生侵袭转移的始动环节[10]。EMT是指具有极性的细胞通过特定程序转化为具有间质表型的生物学过程。通过EMT,细胞失去极性,失去与基底膜的连接表型,获得较高的迁移与侵袭、抗凋亡和降解细胞外基质的能力等间质表型。EMT发生过程中表皮细胞标志物E-cadherin、角蛋白等表达下调或缺失,间质细胞标志物N-cadherin、vimentin等表达则显著上调[11]。EMT是黑素瘤细胞的常见现象,是导致侵袭与转移的重要原因[12]。但目前关于黑素瘤EMT转化的分子机制尚未完全阐明。
近年来研究发现,miRNA参与调控黑素瘤EMT,原癌基因KRAS可通过诱导JUN和SP-1基因而抑制miR-200表达,而miR-200过表达可以逆转KRAS诱导的EMT[7, 13]。MiR-211和miR-542-3p在黑素瘤发生、发展中呈现抑癌基因的特征,分别通过与原癌基因RAB22A和PIM1的3′UTR序列结合抑制这两种原癌基因的表达,进而抑制黑素瘤EMT[7, 14]。MiR-205在多种肿瘤中被发现具有抑癌基因特征,如在乳腺癌[15]、肝癌[16]、胃癌[17]、结肠癌[18]和前列腺癌[19]等肿瘤中其通常处于低表达水平,这可能与miR-205基因转录启动子过度甲基化有关。黑素瘤转移灶组织中miR-205的表达低于原发性黑素瘤,提示其表达减低与黑素瘤侵袭转移有关,在黑素瘤细胞中过表达miR-205能抑制其增殖、多克隆形成和动物体内肿瘤形成等,被认为是黑素瘤的抑癌基因[20]。本研究发现miR-205在A375和A2058两种细胞株中表达几乎缺失,这与他人研究所发现的结果相仿,说明miR-205在黑素瘤组织和细胞中异常低表达,但是至今尚无报道揭示其表达降低的机制。
研究发现miR-205可以通过调控EMT而影响肿瘤细胞的侵袭和转移[21-22]。本研究通过划痕实验和Transwell实验证明miR-205过表达可以抑制A375和A2058细胞的迁移和侵袭能力,并且通过形态学检查发现miR-205过表达后A375和A2058细胞形态从基质型向表皮型转化,且蛋白质印迹法结果显示miR-205过表达可以抑制Twist蛋白表达。Twist蛋白可以与E-cadherin基因启动子结合,抑制E-cadherin基因转录,是促进肿瘤细胞发生EMT的重要蛋白[23]。本研究结果显示Twist蛋白表达降低,其原因可能是miR-205促进表皮型相关蛋白E-cadherin表达的重要原因,同时miR-205抑制间质型相关蛋白vimentin和intergrin的表达,这些发现都从分子水平证明miR-205可以抑制黑素瘤细胞EMT。本研究还观察到miR-205过表达可以抑制MMP-2和MMP-9的表达。MMP-2和MMP-9属于分泌性蛋白,肿瘤细胞过度分泌可以促进细胞外基质降解,为肿瘤侵袭转移提供条件[24]。以上结果说明,miR-205可以通过抑制EMT降低MMP-2和MMP-9蛋白表达,从而抑制黑素瘤细胞的迁移和侵袭。但黑素瘤中miR-205表达几乎缺失,几乎失去抑制EMT的能力,导致肿瘤细胞发生EMT,MMP-2和MMP-9分泌增强,细胞迁移和侵袭能力增强,最终导致黑素瘤向周围组织和器官侵犯转移。
本研究初步发现miR-205在黑素瘤发展过程中具有抑癌基因的特征,miR-205过表达可以抑制黑素瘤细胞的迁移和侵袭能力,且miR-205过表达可以抑制EMT,抑制MMP-2和MMP-9等蛋白表达,进而抑制肿瘤的侵袭转移。在下一步研究中,我们将对miR-205在黑素瘤组织和细胞中表达降低和miR-205抑制Twist等基因表达的分子机制进行探讨,以期阐明黑素瘤侵袭转移的分子机制,为黑素瘤的靶向治疗提供新型有效的潜在靶点。
| [1] | FERLAY J, SHIN H R, BRAY F, FORMAN D, MATHERS C, PARKIN D M. Estimates of worldwide burden of cancer in 2008:GLOBOCAN 2008[J]. Int J Cancer, 2010, 127: 2893–2917. DOI: 10.1002/ijc.v127:12 |
| [2] | HAQQ C, NOSRATI M, SUDILOVSKY D, CROTHERS J, KHODABAKHSH D, PULLIAM B L, et al. The gene expression signatures of melanoma progression[J]. Proc Natl Acad Sci USA, 2005, 102: 6092–6097. DOI: 10.1073/pnas.0501564102 |
| [3] | KALLURI R, WEINBERG R A. The basics of epithelial-mesenchymal transition[J]. J Clin Invest, 2009, 119: 1420–1428. DOI: 10.1172/JCI39104 |
| [4] | WEHBE M, SOUDJA S M, MAS A, CHASSON L, GUINAMARD R, DE TENBOSSCHE C P, et al. Epithelial-mesenchymal-transition-like and TGFbeta pathways associated with autochthonous inflammatory melanoma development in mice[J/OL]. PLoS One, 2012, 7:e49419. doi:10.1371/journal.pone.0049419. |
| [5] | VENTURA A, JACKS T. MicroRNAs and cancer:short RNAs go a long way[J]. Cell, 2009, 136: 586–591. DOI: 10.1016/j.cell.2009.02.005 |
| [6] | SU B B, ZHOU S W, GAN C B, ZHANG X N. MiR-330-5p regulates tyrosinase and PDIA3 expression and suppresses cell proliferation and invasion in cutaneous malignant melanoma[J]. J Surg Res, 2016, 203: 434–440. DOI: 10.1016/j.jss.2016.03.021 |
| [7] | RANG Z, YANG G, WANG Y W, CUI F. miR-542-3p suppresses invasion and metastasis by targeting the proto-oncogene serine/threonine protein kinase, PIM1, in melanoma[J]. Biochem Biophys Res Commun, 2016, 474: 315–320. DOI: 10.1016/j.bbrc.2016.04.093 |
| [8] | SALAJEGHEH A, DOLAN-EVANS E, SULLIVAN E, IRANI S, RAHMAN M A, VOSGHA H, et al. The expression profiles of the galectin gene family in primary and metastatic papillary thyroid carcinoma with particular emphasis on galectin-1 and galectin-3 expression[J]. Exp Mol Pathol, 2014, 96: 212–218. DOI: 10.1016/j.yexmp.2014.02.003 |
| [9] | ISHIYAMA N, LEE S H, LIU S, LI G Y, SMITH M J, REICHARDT L F, et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion[J]. Cell, 2010, 141: 117–128. DOI: 10.1016/j.cell.2010.01.017 |
| [10] | OMBRATO L, MALANCHI I. The EMT universe:space between cancer cell dissemination and metastasis initiation[J]. Crit Rev Oncog, 2014, 19: 349–361. DOI: 10.1615/CritRevOncog.v19.i5 |
| [11] | McDONALD T M, PASCUAL A S, UPPALAPATI C K, COOPER K E, LEYVA K J, HULL E E. Zebrafish keratocyte explant cultures as a wound healing model system:differential gene expression & morphological changes support epithelial-mesenchymal transition[J]. Exp Cell Res, 2013, 319: 1815–1827. DOI: 10.1016/j.yexcr.2013.03.036 |
| [12] | SCHLEGEL N C, VON PLANTA A, WIDMER D S, DUMMER R, CHRISTOFORI G. PI3K signalling is required for a TGFβ-induced epithelial-mesenchymal-like transition (EMT-like) in human melanoma cells[J]. Exp Dermatol, 2015, 24: 22–28. DOI: 10.1111/exd.2015.24.issue-1 |
| [13] | ZHONG X, ZHENG L, SHEN J, ZHANG D, XIONG M, ZHANG Y, et al. Suppression of microRNA 200 family expression by oncogenic KRAS activation promotes cell survival and epithelial-mesenchymal transition in KRAS-driven cancer[J]. Mol Cell Biol, 2016, 36: 2742–2754. DOI: 10.1128/MCB.00079-16 |
| [14] | YU H, YANG W. MiR-211 is epigenetically regulated by DNMT1 mediated methylation and inhibits EMT of melanoma cells by targeting RAB22A[J]. Biochem Biophys Res Commun, 2016, 476: 400–405. DOI: 10.1016/j.bbrc.2016.05.133 |
| [15] | HU Y, QIU Y, YAGUE E, JI W, LIU J, ZHANG J. MiRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer[J/OL]. Cell Death Dis, 2016, 7:e2291. doi:10.1038/cddis.2016.194. |
| [16] | ZHANG T, ZHANG J, CUI M, LIU F, YOU X, DU Y, et al. Hepatitis B virus Ⅹ protein inhibits tumor suppressor miR-205 through inducing hypermethylation of miR-205 promoter to enhance carcinogenesis[J]. Neoplasia, 2013, 15: 1282–1291. DOI: 10.1593/neo.131362 |
| [17] | XU C, LI M, ZHANG L, BI Y, WANG P, LI J, et al. MicroRNA-205 suppresses the invasion and epithelial-mesenchymal transition of human gastric cancer cells[J]. Mol Med Rep, 2016, 13: 4767–4773. |
| [18] | NGUYEN-VU T, WANG J, MESMAR F, MUKHOPADHYAY S, SAXENA A, McCOLLUM C W, et al. Estrogen receptor beta reduces colon cancer metastasis through a novel miR-205-PROX1 mechanism[J]. Oncotarget, 2016, 7: 42159–42171. |
| [19] | XU C G, YANG M F, FAN J X, WANG W. MiR-30a and miR-205 are downregulated in hypoxia and modulate radiosensitivity of prostate cancer cells by inhibiting autophagy via TP53INP1[J]. Eur Rev Med Pharmacol Sci, 2016, 20: 1501–1508. |
| [20] | DAR A A, MAJID S, DE SEMIR D, NOSRATI M, BEZROOKOVE V, KASHANI-SABET M. MiRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein[J]. J Biol Chem, 2011, 286: 16606–16614. DOI: 10.1074/jbc.M111.227611 |
| [21] | GREGORY P A, BRACKEN C P, BERT A G, GOODALL G J. MicroRNAs as regulators of epithelial-mesenchymal transition[J]. Cell Cycle, 2008, 7: 3112–3118. DOI: 10.4161/cc.7.20.6851 |
| [22] | LAMOUILLE S, SUBRAMANYAM D, BLELLOCH R, DERYNCK R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs[J]. Curr Opin Cell Biol, 2013, 25: 200–207. DOI: 10.1016/j.ceb.2013.01.008 |
| [23] | MONTSERRAT N, GALLARDO A, ESCUIN D, CATASUS L, PRAT J, GUTIERREZ-AVIGNO F J, et al. Repression of E-cadherin by SNAIL, ZEB1, and TWIST in invasive ductal carcinomas of the breast:a cooperative effort?[J]. Hum Pathol, 2011, 42: 103–110. DOI: 10.1016/j.humpath.2010.05.019 |
| [24] | HUNG W C, TSENG W L, SHIEA J, CHANG H C. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells[J]. Cancer Lett, 2010, 288: 156–161. DOI: 10.1016/j.canlet.2009.06.032 |
 2017, Vol. 38
2017, Vol. 38


