非酒精性脂肪肝病(NAFLD)指排除酒精、病毒及其他明确的肝脏损害因素,由遗传、环境、代谢应激相关因素共同导致的脂质在肝细胞沉积,进而引起弥漫性肝细胞脂肪变。NAFLD发展到后期会出现肝纤维化、肝硬化,甚至肝细胞癌。现今NAFLD作为一种越来越普遍的慢性肝病,日益受到医务工作者的重视[1]。
大麻的药用价值虽然早就为人们所熟知,但因为其成瘾性和精神致幻作用,使其临床应用受到极大限制。大麻主要的活性成分是Δ9-四氢大麻醇和大麻二酚(cannabidiol,CBD),与Δ9-四氢大麻醇不同的是,CBD没有成瘾性和精神致幻作用[2]。CBD可以通过受体和非受体的方式发挥药理作用,目前关于CBD的研究主要集中于免疫调控、促血管新生、抗癌,以及神经和心血管保护作用等[3],而在肝功能保护方面的研究较少。体外细胞实验发现,Δ9-四氢大麻醇或CBD可以减轻油酸(不饱和脂肪酸)在肝细胞中的聚集[4],预示CBD也许可以减轻NAFLD发病过程中脂肪酸对肝脏细胞造成的损伤。研究证明NAFLD中主要是饱和脂肪酸诱发脂肪毒性,促进细胞凋亡[5-6]。为了证实CBD在NAFLD中的潜在作用,需要进一步阐明CBD是否对饱和脂肪酸诱导的肝脏损伤具有保护作用。
棕榈酸(palmitic acid,PA)是一种饱和脂肪酸,常被用于在体外实验中模拟NAFLD发病过程,诱导肝细胞损伤[7]。因此本研究建立PA诱导的肝细胞损伤模型,观察CBD对PA在肝细胞中诱导的脂肪毒性的作用,并探讨相关机制。
1 材料和方法 1.1 实验动物15只清洁级8周龄SD大鼠(250~300 g)购自第二军医大学动物实验中心,性别不限。动物生产许可证号:SCXK(沪)2012-0003,使用许可证号:SYXK(沪)2012-0003。
1.2 原代大鼠肝细胞培养大鼠腹腔注射3%戊巴比妥钠(2 mL/kg)麻醉,固定、消毒,U型剖腹,18号留置针穿刺门静脉,用37 ℃ Hank’s平衡盐溶液冲洗肝脏,然后用37 ℃预温的含有Ⅳ型胶原酶Hank’s平衡盐溶液(0.75 mg/mL,5 mL/min)灌流,摘取肝脏,于4 ℃预冷的DMEM培养液中剪碎。细胞悬液先后经过70 μm和40 μm的尼龙筛网过滤。4 ℃ 30×g离心5 min,取沉淀用DMEM溶液洗2次。计数后以5×105/mL细胞密度接种、培养(10%胎牛血清的DMEM培养液,37 ℃、5% CO2、95%空气的培养箱),约6 h后细胞贴壁,换液,培养24 h后开始实验。
1.3 实验分组与处理首先分别给予肝细胞1 μmol/L和5 μmol/L的CBD处理24 h,对照组(0 μmol/L)给予等体积0.03% DMSO处理相同时间,每组设3个复孔,以观察CDB对肝细胞自噬流的影响。
另取肝细胞分为4组:阴性对照组、PA组、PA+CBD组和PA+CBD+CQ组。(1) PA组,给予肝细胞PA (800 μmol/L[7];P0500,美国Sigma公司)处理24 h;(2) PA+CBD组,给予肝细胞800 μmol/L PA与5 μmol/L[8] CBD (C7515,美国Sigma公司)处理24 h;(3) PA+CBD+CQ组,给予肝细胞PA (800 μmol/L)、CBD (5 μmol/L)和自噬抑制剂氯喹(chloroquine,CQ;50 nmol/L[7];C6628,美国Sigma公司)处理24 h;(4) 阴性对照组,给予肝细胞与前3组等体积0.03% DMSO处理细胞24 h。每个实验组DMSO终浓度均为0.03%。每组设3个复孔。
1.4 蛋白质印迹法检测自噬相关蛋白的表达加入细胞裂解液(P0013,上海碧云天生物试剂公司)裂解细胞并收集细胞蛋白,100 ℃蛋白变性10 min,用蛋白浓度定量检测试剂盒(GMS30031,上海杰美基因医药科技有限公司)测定蛋白浓度。行SDS-PAGE分离蛋白,湿转法转膜(PVDF膜),5%脱脂奶粉室温封闭4 h,TBST溶液洗涤后依次标记一抗LC3抗体(1:1 000;L7543,美国Sigma公司)、p62抗体(1:500;SC-28359,美国Santa Cruz公司)和相应二抗,以GAPDH作为内参照蛋白(GAPDH抗体,SC-32233,美国Santa Cruz公司)。采用ECL发光法显影拍照,用Image J图像分析软件分析条带灰度值。
1.5 流式细胞术检测细胞凋亡水平按照细胞凋亡检测试剂盒(V13241,美国Invitrogen公司)操作说明,收集各组细胞,800×g离心8 min,PBS冲洗2次。加入膜联蛋白Ⅴ(annexin Ⅴ)和碘化丙啶(propidium iodide,PI),混匀后25 ℃避光孵育15 min。用流式细胞仪检测各组细胞的凋亡情况,计算凋亡细胞的比例。
1.6 实时定量PCR(qPCR)检测内质网应激相关基因mRNA的表达采用TRIzol法提取细胞总RNA,反转录获得cDNA,按qPCR试剂盒(210210,德国QIAGEN公司)操作说明进行PCR反应,检测CCAAT/增强子结合蛋白同源蛋白(CHOP;上游5′-GCA TGA AGG AGA AGG AGC AG-3′,下游5′-CTT CCG GAG AGA CAG ACA GG-3′)、葡萄糖调节蛋白78(GRP78;上游5′-TCA TCG GAC GCA CTT GGA A-3′,下游5′-CAA CCA CCT TGA ATG GCA AGA-3′)和X盒结合蛋白1(XBP-1;上游5′-CCT GAG CCC GGA GGA GAA-3′,下游5′-CTC GAG CAG TCT GCG CTG-3′)mRNA的表达。用GAPDH(5′-AGA CAG CCG CAT CTT CTT GT-3′;5′-CTT GCC GTG GGT AGA GTC AT-3′)作为内参基因。采用MJ Opticon Monitor软件记录整理所得数据,mRNA表达水平用倍数变化(2-ΔΔCt)表示。
1.7 探针法检测线粒体功能收集细胞,加入预冷的提取液(0.25 mol/L蔗糖,0.01 mol/L Tris-HCl,0.5 mmol/L EDTA,0.1%牛血清白蛋白;pH 7.4)。差速离心法分离纯化线粒体。使用Rh123探针(R233,上海同仁化学研究所)检测线粒体膜电位,激发波长/发射波长为488 nm/525 nm。以lucigenin(ENZ-52154,广州宝柏贸易有限公司)为探针检测线粒体的活性氧簇(reactive oxygen species, ROS)生成水平,激发波长/发射波长为455nm/505 nm。
1.8 统计学处理应用SPSS 12.0软件进行数据分析。实验数据以x±s表示。多组间比较采用单因素方差分析(one-way ANOVA);方差齐性时运用LSD方法里德多重比较,方差不齐性时则运用Dunnett’s T3法。检验水准(α)为0.05。
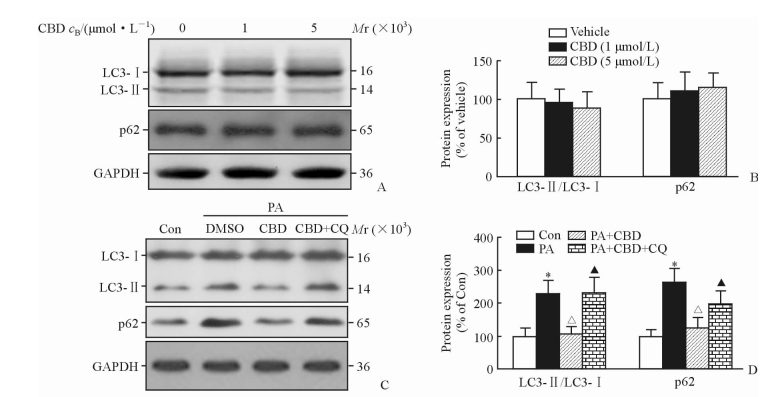
2 结果 2.1 CBD对PA刺激的肝细胞的自噬流影响由图 1A、1B可见,单独使用不同剂量的CBD处理原代培养的肝细胞,均不影响肝细胞中LC3-Ⅱ/LC3-Ⅰ的比值和p62的蛋白表达(P>40.05)。
|
图 1 CBD对PA刺激的肝细胞自噬流的影响 Fig 1 Effect of CBD on autophagic flux of hepatocytes stimulated with PA A, B: Expression of LC3 and p62 treated with CBD; C, D: Expression of LC3 and p62 stimulated with PA. CBD: Cannabidiol; LC3: Autophagy-related protein microtubule-associated protein 1 light chain 3; GAPDH: Glyceraldehyde phosphate dehydrogenase; Con: Control; PA: Palmitic acid; CQ: Chloroquine. *P < 0.05 vs Con group; ΔP < 0.05 vs PA group; ▲P < 0.05 vs PA+CBD group. n=3, x±s |
给予肝细胞PA刺激增加了肝细胞内LC3-Ⅱ/LC3-Ⅰ的比值和p62的蛋白表达(P<0.05),提示PA刺激使肝细胞内自噬溶酶体活性增加,降解速度降低,自噬流受到破坏。给予CBD处理后肝细胞内LC3-Ⅱ/LC3-Ⅰ的比值和p62的蛋白表达均降低(P<0.05),即CBD能够促进PA刺激诱导的肝细胞内自噬溶酶体的降解,促进了自噬流。而同时给予自噬抑制剂CQ处理肝细胞后,CBD对自噬流的促进作用被逆转(P<0.05)。见图 1C、1D。
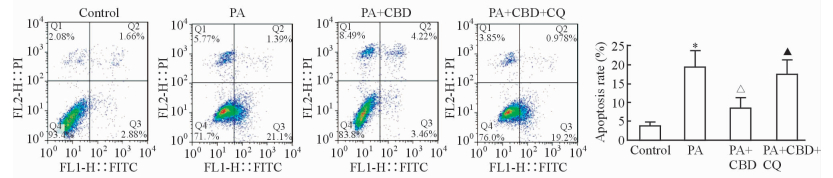
2.2 CBD对PA刺激的肝细胞凋亡的影响如图 2所示,PA刺激可以增加肝细胞的凋亡(P<0.05),给予CBD处理后肝细胞凋亡数目减少(P<0.05);同时给予自噬抑制剂CQ处理后可以逆转CBD的保护作用(P<0.05)。提示CBD可以通过促进PA刺激的肝细胞内自噬流的发生减轻PA诱导的肝细胞凋亡。
|
图 2 CBD对PA刺激的肝细胞凋亡的影响 Fig 2 Effect of CBD on PA-induced hepatocytes apoptosis CBD: Cannabidiol; PA: Palmitic acid; CQ: Chloroquine. *P < 0.05 vs control group; ΔP < 0.05 vs PA group; ▲P < 0.05 vs PA+CBD group. n=3, x±s |
2.3 CBD对PA刺激的肝细胞内质网应激的影响
由图 3可见,原代培养的肝细胞给予PA刺激后,其内质网应激相关因子CHOP、GRP78和XBP-1的mRNA表达均增加(P<0.05),同时给予CBD处理后CHOP、GRP78和XBP-1的mRNA表达均降低(P<0.05),给予CQ处理后CBD的保护作用减弱(P<0.05)。结果提示CBD通过促进PA诱导的肝细胞的自噬流改善其内质网应激。

|
图 3 CBD对PA刺激的肝细胞内质网应激的影响 Fig 3 Effect of CBD on endoplasmic reticulum stress of hepatocytes stimulated with PA CBD: Cannabidiol; PA: Palmitic acid; CQ: Chloroquine; CHOP: CCAAT/enhancer-binding protein homologous protein; GRP78: Glucose-regulated protein 78; XBP-1: X-box protein 1. *P < 0.05 vs control group; ΔP < 0.05 vs PA group; ▲P < 0.05 vs PA+CBD group. n=3, x±s |
2.4 CBD对PA刺激的肝细胞线粒体功能的影响
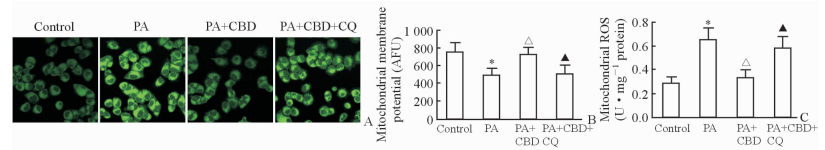
线粒体荧光探针检测图见图 4A。原代培养的肝细胞给予PA刺激后线粒体膜电位下降(P<0.05)、线粒体ROS生成增加(P<0.05),而给予CBD处理后肝细胞线粒体膜电位增加(P<0.05)、线粒体ROS生成减少(P<0.05),同时给予CQ能够逆转CBD对线粒体功能的保护作用(P<0.05)。见图 4B、4C。结果提示CBD能够通过促进PA刺激肝细胞的自噬流改善PA诱导的肝细胞线粒体功能障碍。
|
图 4 CBD对PA刺激的肝细胞线粒体功能的影响 Fig 4 Effect of CBD on mitochondrial function of hepatocytes stimulated with PA A: Fluorescent images of mitochondrial; B: Change of mitochondrial membrane potential; C: Contents of mitochondrial ROS. CBD: Cannabidiol; PA: Palmitic acid; CQ: Chloroquine; AFU: Arbitrary fluorescence unit; ROS: Reactive oxygen species. Original magnification: ×400 (A). *P < 0.05 vs control group; ΔP < 0.05 vs PA group; ▲P < 0.05 vs PA+CBD group. n=3, x±s |
3 讨论
应激状态下细胞自噬清除无用的、多余的、受损的或癌变的细胞器、异常蛋白、脂滴,在调控代谢、维持机体内环境稳态方面发挥重要作用[9]。既往多以自噬体的数量,即LC3-Ⅱ/LC3-Ⅰ的比值评价自噬水平的强弱;最近研究发现自噬体数量的增加并不能从本质上反映自噬水平,因为自噬体的数量由形成和清除两方面决定,准确地评价自噬水平不仅仅是检测自噬体的数量,还应动态观察整个自噬流是否通畅;并认为自噬底物p62的降解对于评价自噬流尤为重要[10]。当自噬体生成增加,但自噬流被破坏时,自噬体的降解水平降低,细胞内自噬体大量累积促使细胞凋亡发生[11]。本研究结果显示PA诱导肝细胞内自噬溶酶体活性增加,降解速度降低,使自噬流受到破坏;给予CBD处理降低了肝细胞内LC3-Ⅱ/LC3-Ⅰ的比值和p62蛋白的表达,即CBD通过促进自噬体的降解减轻PA对自噬流的破坏。本研究结果也显示CBD不影响正常肝脏细胞中LC3-Ⅱ/LC3-Ⅰ的比值和p62蛋白的表达。而研究发现CBD对多种疾病模型的自噬流有促进作用,如在tau病小鼠模型中,CBD可以增加自噬流的发生,减少体内tau蛋白的沉积,从而减轻症状[12];在毛果芸香碱诱发的癫疒间发作慢性期,CBD可以促进自噬流的发生,从而减轻癫疒间症状[13];在酒精诱发的小鼠脂肪肝中,CBD也可以促进肝脏中自噬流的发生,从而减轻肝脏损伤[14]。我们前期研究发现促进自噬流不仅可以减少肝细胞内脂质沉积,还可以减轻内质网应激和线粒体损伤,从而缓解蛋氨酸/胆碱缺乏饲料诱发小鼠脂肪性肝脏病变[15],可见CBD恢复PA破坏的自噬流对保护PA诱导的肝细胞损伤十分重要。
内质网是细胞内蛋白合成、折叠、成熟与分泌的主要场所。在疾病状态下,蛋白质因某些因素不能正常修饰或蛋白质由内质网向高尔基体转运受阻时, 导致未折叠蛋白或错误折叠蛋白在内质网腔大量蓄积, 继而破坏钙离子平衡,造成典型的内质网应激。肝脏细胞内血浆蛋白的合成与分泌、脂蛋白和极低密度脂蛋白的合成与分泌、胆固醇的合成及外源物代谢等都需要内质网参与完成,异常导致内质网应激也参与多种疾病的发生、发展,如在非酒精性脂肪性肝炎中,内质网应激促进了肝细胞内脂肪沉积、炎症反应、氧化应激、细胞凋亡的发生[16]。本研究结果表明PA刺激下肝细胞内内质网应激水平上调,而CBD处理能够通过促进自噬流减轻PA诱导的内质网应激。
线粒体是细胞内ROS生成的主要亚细胞器,线粒体损伤时膜电位下降、膜通透性增加、ROS生成增加,同时线粒体中细胞色素c等因子释放进入细胞浆引发线粒体依赖的细胞凋亡发生;而自噬对维持线粒体功能的正常十分重要,通过清除细胞内过多或受损的线粒体维持细胞内线粒体的数目和功能[17]。研究表明线粒体功能损伤在NAFLD中发挥重要作用[18],而使用花生四烯酸、丁硫氨酸亚砜胺或氯仿诱导的HepG2肝细胞线粒体损伤能够被自噬激动剂改善[19]。本研究结果也显示PA刺激导致肝细胞内线粒体膜电位下降、ROS生成增加,受损肝细胞线粒体功能异常,而给予细胞CBD处理能够通过促进自噬流减轻PA诱导的线粒体损伤,降低了线粒体损伤相关的氧化应激和细胞凋亡。
综上所述,在离体原代培养的大鼠肝细胞中,CBD能够通过促进自噬流减轻PA诱导的肝细胞凋亡,改善受损肝细胞的内质网应激和线粒体功能异常。
| [1] | HUANG X, XU M, CHEN Y, PENG K, HUANG Y, WANG P, et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle-aged and elderly Chinese[J/OL]. Medicine (Baltimore), 2015, 94:e1682. doi:10.1097/MD.0000000000001682. |
| [2] | JACOBS D S, KOHUT S J, JIANG S, NIKAS S P, MAKRIYANNIS A, BERGMAN J. Acute and chronic effects of cannabidiol on Δ9-tetrahydrocannabinol (Δ < 9-THC)-induced disruption in stop signal task performance[J]. Exp Clin Psychopharmacol, 2016, 24: 320–330. DOI: 10.1037/pha0000081 |
| [3] | PISANTI S, MALFITANO A M, CIAGLIA E, LAMBERTI A, RANIERI R, CUOMO G, et al. Cannabidiol:state of the art and new challenges for therapeutic applications[J/OL]. Pharmacol Ther, 2017. doi:10.1016/j.pharmthera.2017.02.041. |
| [4] | SILVESTRI C, PARIS D, MARTELLA A, MELCK D, GUADAGNINO I, CAWTHORNE M, et al. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis[J]. J Hepatol, 2015, 62: 1382–1390. DOI: 10.1016/j.jhep.2015.01.001 |
| [5] | BARREYRO F J, KOBAYASHI S, BRONK S F, WERNEBURG N W, MALHI H, GORES G J. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis[J]. J Biol Chem, 2007, 282: 27141–27154. DOI: 10.1074/jbc.M704391200 |
| [6] | MALHI H, BRONK S F, WERNEBURG N W, GORES G J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis[J]. J Biol Chem, 2006, 281: 12093–12101. DOI: 10.1074/jbc.M510660200 |
| [7] | GONZÁLEZ-RODRÍGUEZ A, MAYORAL R, AGRA N, VALDECANTOS M P, PARDO V, MIQUILENA-COLINA M E, et al. Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD[J/OL]. Cell Death Dis, 2014, 5:e1179. doi:10.1038/cddis.2014.162. |
| [8] | SUN S, HU F, WU J, ZHANG S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons[J]. Redox Biol, 2017, 11: 577–585. DOI: 10.1016/j.redox.2016.12.029 |
| [9] | ANDING A L, BAEHRECKE E H. Autophagy in cell life and cell death[J]. Curr Top Dev Biol, 2015, 114: 67–91. DOI: 10.1016/bs.ctdb.2015.07.012 |
| [10] | ZHANG X J, CHEN S, HUANG K X, LE W D. Why should autophagic flux be assessed?[J]. Acta Pharmacol Sin, 2013, 34: 595–599. DOI: 10.1038/aps.2012.184 |
| [11] | OUYANG L, SHI Z, ZHAO S, WANG F T, ZHOU T T, LIU B, et al. Programmed cell death pathways in cancer:a review of apoptosis, autophagy and programmed necrosis[J]. Cell Prolif, 2012, 45: 487–498. DOI: 10.1111/cpr.2012.45.issue-6 |
| [12] | CASAREJOS M J, PERUCHO J, GOMEZ A, MUÑOZ M P, FERNANDEZ-ESTEVEZ M, SAGREDO O, et al. Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy[J]. J Alzheimers Dis, 2013, 35: 525–539. |
| [13] | HOSSEINZADEH M, NIKSERESHT S, KHODAGHOLI F, NADERI N, MAGHSOUDI N. Cannabidiol post-treatment alleviates rat epileptic-related behaviors and activates hippocampal cell autophagy pathway along with antioxidant defense in chronic phase of pilocarpine-induced seizure[J]. J Mol Neurosci, 2016, 58: 432–440. DOI: 10.1007/s12031-015-0703-6 |
| [14] | YANG L, ROZENFELD R, WU D, DEVI L A, ZHANG Z, CEDERBAUM A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy[J]. Free Radic Biol Med, 2014, 68: 260–267. DOI: 10.1016/j.freeradbiomed.2013.12.026 |
| [15] | CHEN R, WANG Q, SONG S, LIU F, HE B, GAO X. Protective role of autophagy in methionine-choline deficient diet-induced advanced nonalcoholic steatohepatitis in mice[J]. Eur J Pharmacol, 2016, 770: 126–133. DOI: 10.1016/j.ejphar.2015.11.012 |
| [16] | BOZAYKUT P, SAHIN A, KARADEMIR B, OZER N K. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis[J]. Mech Ageing Dev, 2016, 157: 17–29. DOI: 10.1016/j.mad.2016.07.001 |
| [17] | LIU K, ZHAO Q, LIU P, CAO J, GONG J, WANG C, et al. ATG3-dependent autophagy mediates mitochondrial homeostasis in pluripotency acquirement and maintenance[J]. Autophagy, 2016, 12: 2000–2008. DOI: 10.1080/15548627.2016.1212786 |
| [18] | CABRÉ N, CAMPS J, JOVEN J. Inflammation, mitochondrial metabolism and nutrition:the multi-faceted progression of non-alcoholic fatty liver disease to hepatocellular carcinoma[J]. Hepatobiliary Surg Nutr, 2016, 5: 438–443. DOI: 10.21037/hbsn |
| [19] | WU D, CEDERBAUM A I. Inhibition of autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via elevated oxidative stress, mitochondria dysfunction and activation of p38 and JNK MAPK[J]. Redox Biol, 2013, 1: 552–565. DOI: 10.1016/j.redox.2013.10.008 |
 2017, Vol. 38
2017, Vol. 38


