在糖尿病患者中,甲状腺功能异常的发生率居高不下,其中以甲状腺功能减退(以下简称“甲减”)最为常见[1]。一项基于1 310例糖尿病患者的大型临床研究显示,甲状腺功能障碍的总体患病率为13.4%,其中包括8.8%的男性和16.8%的女性,明显高于基础人群的患病率(6.6%)[2]。二甲双胍(metformin)是经典的降糖药物之一,但除了降糖作用以外,其还可用于治疗多囊卵巢综合征(polycystic ovary syndrome,PCOS)[3]、肿瘤[4]、非酒精性脂肪性肝炎[5]等。研究表明二甲双胍能降低糖尿病合并甲减或者亚临床甲减患者的促甲状腺激素(thyroid stimulating hormone,TSH)水平[6-8],另有研究发现服用二甲双胍的糖尿病患者形成甲状腺肿和甲状腺结节的风险低于未服用患者[9]。
目前,二甲双胍对甲状腺功能的研究仅限于有限的临床资料研究,而对于二甲双胍对甲状腺激素改变、形态及其机制尚缺乏动物实验研究。二甲双胍作用的细胞和分子机制是非常复杂和多方面的,但普遍认为二甲双胍诱导的腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)的激活在诸多效应中起了重要作用[10-11]。而近年来有细胞实验表明AMPK通路能影响甲状腺的钠碘同向转运体(sodium/iodide symporter,NIS)水平和碘摄取[12-13],碘的摄取对于甲状腺激素的合成至关重要。本实验通过构建甲减的大鼠模型,观察二甲双胍灌胃对甲减大鼠甲状腺激素水平的影响,并通过检测甲状腺NIS表达、观察甲状腺组织H-E染色情况等初步探讨其影响机制,为临床上二甲双胍应用于甲减的辅助治疗或者甲状腺肿瘤术后的TSH抑制治疗提供依据。
1 材料和方法 1.1 动物取3个月龄雄性SD大鼠36只,体质量(297.06±12.71) g,同批次购自上海斯莱克实验动物有限公司,饲养于第二军医大学药学院实验动物房[动物生产许可证号:SCXK(沪)2012-0003;动物使用许可证号:SYXK(沪)2012-0003],给予12 h/12 h昼夜节律,不限制摄食和饮水,每笼6只。室温控制在20~25 ℃,湿度控制在55%左右。
1.2 动物模型的建立与分组处理大鼠适应性饮水摄食3 d后,按体质量从低到高排序编号:1~36号,然后根据在线随机种子数随机分组为正常对照(NC组)、甲减大鼠模型组(HT组)和二甲双胍组(Met组)。对HT组和Met组大鼠进行甲减造模,方法如下:将氨基三唑(AMT;美国Sigma公司)按质量分数0.01%溶于饮用水,喂养大鼠4周时于眼眶取血,检测血清T3、T4水平以验证造模是否成功。造模成功后,HT组和Met组饮用水改为0.005% AMT维持。NC组大鼠同期喂养普通饮用水。然后Met组大鼠灌胃给予1 mL/100 g二甲双胍溶液[将20 mg二甲双胍(中美上海施贵宝制药有限公司, 批号:国药准字H20023371) 溶解于1 mL 5%羟甲基纤维素钠(CMC-Na;美国Sigma公司)溶液];HT组和NC组灌胃给予等体积的0.05% CMC-Na。
1.3 甲状腺、下丘脑标本采集大鼠腹腔注射10%水合氯醛麻醉,股动脉放血处死大鼠后,剪开大鼠颈部皮肤,用镊子分开肌肉,可见甲状腺位于气管两侧,将甲状腺及气管剪下立即拍照,然后摘除甲状腺保存于-80 ℃备用或固定行H-E染色。断头后剪除颅顶及侧壁骨质,充分显露脑组织,将鼠脑翻转,可见位于脑底中心、与周围组织界限清楚、米粒大小的菱形区域,即下丘脑,用镊子小心摘取保存于-80 ℃备用。
1.4 甲状腺组织H-E染色在灌胃8周后每组随机取3只大鼠,按照1.3项方法摘取甲状腺后,用10%甲醛溶液固定2 d,逐级脱水,二甲苯透明处理。在恒温箱里石蜡包埋约30 min后,予-20 ℃冰冻切片机4~7 μm厚切片。然后置于65 ℃恒温箱烤片30 min,再依次脱蜡、逐级水化,用纯水冲洗10 min。苏木精液染色5 min,伊红染色2 min,最后用二甲苯透明,65 ℃恒温箱放置15 min后通过显微镜观察拍照。
1.5 电化学发光法检测大鼠血清T3、T4水平分别于灌胃第8周和第12周后处死大鼠(每组6只),股动脉放血取血,离心后的血清采用德国罗氏CobasE602型全自动电化学发光分析仪检测T3、T4水平。T3、T4正常参考值范围分别是1.3~3.1 nmol/L、66.00~181.00 nmol/L。
1.6 qPCR检测大鼠下丘脑促甲状腺激素释放激素前体(proTRH)和甲状腺NIS mRNA表达于灌胃第8周和第12周末摘取大鼠下丘脑和甲状腺组织,采用TRIzol法提取样品总RNA,反转录合成cDNA,以β-actin作为内参照基因,采用qPCR试剂盒(南京诺唯赞生物科技有限公司)检测大鼠下丘脑proTRH和甲状腺NIS mRNA表达。反应条件:95 ℃ 5 min;95 ℃ 15 s、56 ℃ 20 s、72 ℃ 35 s,循环40次。引物序列为proTRH:F 5′-GAC CCT GGA TTC GGG AGT AT-3′,R 5′-CAT CCT GGA GTC TGC GAA GTG-3′;NIS:F 5′-GAG CCA CCA ACG CTT CCA AC-3′,R 5′-AGG TCC CAC CAC AGC AAT CC-3′;β-actin:F 5′-GCT ATG TTG CCC TAG ACT TCG A-3′,R 5′-GAT GCC ACA GGA TTC CAT ACC-3′。所得数据用ABI Prism 7300 SDS软件整理,采用2-ΔΔCt方法进行半定量分析。
1.7 蛋白质印迹法检测大鼠甲状腺组织NIS蛋白表达分离甲状腺组织,剪碎、匀浆,提取组织样品,BCA法行蛋白定量,PAGE胶分离蛋白,转膜,5%脱脂奶粉封闭1 h,分别加入稀释好的NIS抗体(1:1 000;A9605,美国ABclonal公司)和内参照蛋白GAPDH抗体(1:1 000;5174,美国CST公司)4 ℃孵育过夜,TBST洗膜3次,每次5 min,加二抗孵育,洗膜后显影拍照。用Image J图像分析软件测定灰度值并进行半定量分析。
1.8 统计学处理应用SPSS 19.0软件进行数据分析。计量资料若符合正态分布则用x±s表示;若不符合正态分布,则用中位数(Q1,Q3)表示。正态分布的计量资料两组间比较采用t检验;正态分布且方差齐性时多组间比较采用单因素方差分析(one-way ANOVA),并采用SNK检验进行两两比较;若非正态分布或者方差不齐时则用非参数检验。检验水准(α)为0.05。
2 结果 2.1 一般情况NC组大鼠毛发整齐,有光泽,体质量随时间增加。HT组和Met组大鼠饮用含0.01% AMT水4周后体质量几乎没有增加,且毛发稍杂乱,色泽轻微晦暗,精神较差。造模后称量体质量显示HT组、Met组大鼠体质量均低于NC组大鼠(P<0.05),而HT组与Met组差异无统计学意义(P>0.05)。灌胃给药8周和12周后,HT组、Met组大鼠体质量均仍低于NC组大鼠(P<0.05),HT组与Met组差异无统计学意义(P>0.05)。见表 1。
|
|
表 1 各组大鼠不同时间点的体质量 Tab 1 Body mass of rats at different time points in each group |
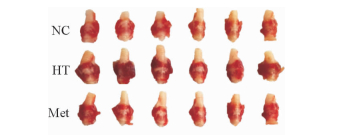
2.2 二甲双胍减轻甲减大鼠的甲状腺肿大
如图 1所示,灌胃12周后NC组大鼠甲状腺颜色淡红,峡部菲薄,两侧甲状腺对称而体积正常;HT组甲状腺颜色暗红,血流丰富,峡部明显增厚,两侧甲状腺不规则肿大;Met组甲状腺较HT组颜色淡,相对HT组峡部较薄,甲状腺肿大较HT组改善。
|
图 1 灌胃12周后各组大鼠甲状腺外观图 Fig 1 Appearance of rat thyroids in each group after intragastric administration for 12 weeks NC: Normal control; HT: Hypothyroidism; Met: Metformin |
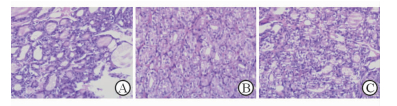
2.3 二甲双胍改善甲减大鼠的甲状腺滤泡上皮增生
H-E染色结果显示NC组滤泡大小不等,内见大量胶质及吸收空泡;HT组大鼠甲状腺滤泡缩小,滤泡上皮成柱状,滤泡周围吸收空泡减少,部分滤泡间纤维组织增生;Met组甲状腺滤泡大小不等,滤泡上皮增生较HT组稍改善(图 2)。
|
图 2 各组大鼠甲状腺组织H-E染色 Fig 2 Thyroid tissues of rats in each group by H-E staining A: Normal control; B: Hypothyroidism; C: Metformin. Original magnification: ×200 |
2.4 二甲双胍升高甲减大鼠的血清T3、T4水平
饮用0.01% AMT 4周后HT组、Met组大鼠血清T3、T4水平均低于NC组(P<0.05)。灌胃8周、12周后HT组大鼠血清T3、T4水平均低于NC组(P<0.05);Met组T3、T4水平均高于HT组(P<0.05)。见图 3。

|
图 3 二甲双胍对甲减大鼠血清T3、T4水平的影响 Fig 3 Effect of metformin intragastric administration on serum T3 and T4 levels of HT rats NC: Normal control; HT: Hypothyroidism; Met: Metformin. i.g.: Intragastric administration. *P < 0.05 vs NC group; △P < 0.05 vs HT group. n=6, x±s |
2.5 二甲双胍对甲减大鼠下丘脑proTRH mRNA及甲状腺NIS的mRNA和蛋白表达的影响
灌胃8周后,HT组(1.66±0.55) 大鼠下丘脑proTRH mRNA表达高于NC组(1.00±0.33),而Met组(1.27±0.54) 表达低于HT组(P均<0.05)。检测大鼠甲状腺组织中NIS mRNA和蛋白表达水平,结果(图 4A~4C)显示灌胃8周时,HT组NIS mRNA水平高于NC组(P<0.05),两组间NIS蛋白表达水平差异无统计学意义,而Met组大鼠甲状腺组织中NIS mRNA和蛋白表达均低于HT组(P<0.05);灌胃12周(图 4D~4F)时,HT组大鼠甲状腺组织中NIS mRNA表达与Met组差异无统计学意义,但Met组NIS蛋白表达高于HT组(P<0.05)。

|
图 4 二甲双胍对甲减大鼠甲状腺NIS mRNA和蛋白表达的影响 Fig 4 Effect of metformin intragastric administration on expression of NIS mRNA and protein in thyroid of HT rats A-C: Intragastric administration for 8 weeks; D-F: Intragastric administration for 12 weeks. NC: Normal control; HT: Hypothyroidism; Met: Metformin. NIS: Sodium/iodide symporter. *P < 0.05 vs NC group; △P < 0.05 vs HT group. n=6 in Fig 4A and 4D; n=3 in Fig 4C and 4F. x±s |
3 讨论
糖尿病合并甲减或亚临床甲减是临床上较为常见的现象,而二甲双胍又是糖尿病患者应用最多的一线口服降糖药物。Vigersky等[14]对3例伴有原发性甲减的糖尿病患者加用口服二甲双胍后,发现TSH水平明显降低,其降低程度与二甲双胍剂量相关,而T3、T4水平无明显变化。后来基于多个临床研究的meta分析也表明二甲双胍能降低糖尿病伴甲减或者亚临床甲减患者的TSH水平,但不改变T3、T4水平[15],其具体机制目前尚不明确。
本实验首先通过将AMT溶于水配成0.01% AMT水喂养大鼠4周,成功构建了甲减模型。AMT构建甲减模型具有周期短、造模方便等优势,是常用的甲减造模方法[16-17]。造模成功后,HT组大鼠精神状态差,体质量下降,毛发无光泽,摄食、饮水量减少,检测甲状腺功能显示T3、T4水平低于正常值。甲状腺外观显示,甲减大鼠甲状腺明显肿大,峡部增厚,颜色加深提示血流丰富,均符合甲减表现。H-E染色结果显示NC组大鼠甲状腺滤泡大小不等,内见大量胶质及吸收空泡;而HT组大鼠甲状腺滤泡缩小,滤泡上皮成柱状,滤泡周围吸收空泡减少,部分滤泡间纤维组织增生,滤泡上皮增生,符合甲减时应对TSH刺激的表现。
对甲减大鼠分别灌胃8周、12周二甲双胍后,Met组大鼠相比HT组T3、T4水平升高,差异均有统计学意义。这不同于以往大多数临床研究提出的二甲双胍仅可以降低甲减患者TSH水平而对T3、T4水平无影响的观点[7-8, 14],我们的动物实验表明二甲双胍还能升高甲减大鼠T3、T4水平,同时降低下丘脑proTRH mRNA表达水平。值得注意的是,Isidro等[6]的前瞻性研究使用二甲双胍干预糖尿病合并甲减的患者后,不仅TSH水平下降,游离T4(FT4) 的平均水平也有所上升,但差异无统计学意义;停用二甲双胍3个月后,FT4水平下降且差异有统计学意义,该研究与我们的结果一致,提示二甲双胍降低TSH水平可能与直接提高甲状腺激素水平有关。特别是近年来报道二甲双胍作为一种AMPK激动剂,能影响甲状腺的NIS蛋白及摄碘功能,更加支持了二甲双胍对甲状腺激素合成的直接效应。甲状腺激素的合成与多种因素相关,包括碘的摄取、酪氨酸的碘化、甲状腺过氧化物酶的活性等。其中NIS介导的碘的摄取是甲状腺激素合成的第一步[18]。2011年Andrade等[12]最早报道AMPK能调节NIS表达和碘摄取,他们使用AMPK激动剂AICAR处理PCCL3大鼠甲状腺滤泡上皮细胞后,其碘摄取和NIS表达均被抑制,而这个效应能被AMPK抑制剂compound C逆转。随后,2014年Abdulrahman等[13]在通过用二甲双胍和compound C处理CFRTL-5大鼠甲状腺滤泡上皮细胞得到了类似的结果,分别能降低和升高NIS水平和摄碘率。
为了探究二甲双胍改善甲状腺功能的原因是否与NIS转录和表达水平相关,我们检测了8周和12周2个不同时间点的NIS mRNA和蛋白水平。我们发现构建大鼠甲减模型后,HT组NIS mRNA表达高于NC组,这与其他文献[19]报道的甲减模型相一致。其NIS mRNA升高原因一方面是甲减后TSH反馈性升高,而TSH是影响NIS表达和定位的重要因素,对NIS基因的表达起到促进作用[20];另外AMT本身可能与丙硫氧嘧啶相似,能在TSH不存在的情况下促进NIS蛋白表达[21]。而用二甲双胍灌胃8周后,Met组NIS mRNA和蛋白水平均较HT组下降,这与Abdulrahman等[13]的细胞实验结果相一致,提示二甲双胍能降低甲状腺NIS的转录和表达水平。但继续延长灌胃时间到12周后,Met组NIS mRNA表达与HT组差异无统计意义,而NIS蛋白表达高于HT组,但灌胃第8周和第12周时Met组T3、T4水平均高于HT组,提示甲减大鼠8周内甲状腺激素的增加并非由NIS表达升高所致,而12周时甲状腺的摄碘功能的提高可能与NIS蛋白表达增加有关。
本实验尚存在一些不足之处。第一,TSH作为一个重要的甲状腺功能指标,不能通过临床所用的电化学发光仪器检测,故本实验缺乏大鼠血清TSH的数据,我们将会在后期实验中用放射免疫检测方法检测这一指标,以进一步明确本实验结论。第二,甲减大鼠二甲双胍灌胃第8周和第12周时得到NIS mRNA和蛋白表达结果不同,这一问现象值得进一步探讨。
二甲双胍作为甲减的补充治疗可能减少甲状腺激素的用量,从而最大限度地减少高剂量甲状腺激素诱导的骨代谢异常和心血管不良反应的发生,值得进一步深入研究,为糖尿病以及甲减的综合治疗提供新的思路和方法。本实验发现二甲双胍能够改变甲减大鼠甲状腺NIS mRNA和蛋白的表达,且能升高甲状腺激素水平,这些结论可能对以后的相关临床工作有着深远意义。
| [1] | KADIYALA R, PETER R, OKOSIEME O E. Thyroid dysfunction in patients with diabetes:clinical implications and screening strategies[J]. Int J Clin Pract, 2010, 64: 1130–1139. DOI: 10.1111/j.1742-1241.2010.02376.x |
| [2] | PERROS P, McCRIMMON R J, SHAW G, FRIER B M. Frequency of thyroid dysfunction in diabetic patients:value of annual screening[J]. Diabet Med, 1995, 12: 622–627. DOI: 10.1111/dme.1995.12.issue-7 |
| [3] | PALOMBA S, FALBO A, JR O F, ZULLO F. Effect of preconceptional metformin on abortion risk in polycystic ovary syndrome:a systematic review and meta-analysis of randomized controlled trials[J]. Fertil Steril, 2009, 92: 1646–1658. DOI: 10.1016/j.fertnstert.2008.08.087 |
| [4] | CURRIE C J, POOLE C D, GALE E A. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes[J]. Diabetologia, 2009, 52: 1766–1777. DOI: 10.1007/s00125-009-1440-6 |
| [5] | UYGUN A, KADAYIFCI A, ISIK A T, OZGURTAS T, DEVECI S, TUZUN A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis[J]. Aliment Pharmacol Ther, 2004, 19: 537–544. DOI: 10.1111/apt.2004.19.issue-5 |
| [6] | ISIDRO M L, PENÍN M A, NEMIÑA R, CORDIDO F. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy[J]. Endocrine, 2007, 32: 79–82. DOI: 10.1007/s12020-007-9012-3 |
| [7] | CAPPELLI C, ROTONDI M, PIROLA I, AGOSTI B, GANDOSSI E, VALENTINI U, et al. TSH-lowering effect of metformin in type 2 diabetic patients:differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients[J]. Diabetes Care, 2009, 32: 1589–1590. DOI: 10.2337/dc09-0273 |
| [8] | FOURNIER J P, YIN H, YU O H, AZOULAY L. Metformin and low levels of thyroid-stimulating hormone in patients with type 2 diabetes mellitus[J]. CMAJ, 2014, 186: 1138–1145. DOI: 10.1503/cmaj.140688 |
| [9] | BLANC E, PONCE C, BRODSCHI D, NEPOTE A, BARRETO A, SCHNITMAN M, et al. Association between worse metabolic control and increased thyroid volume and nodular disease in elderly adults with metabolic syndrome[J]. Metab Syndr Relat Disord, 2015, 13: 221–226. DOI: 10.1089/met.2014.0158 |
| [10] | GIANNARELLI R, ARAGONA M, COPPELLI A, DEL P S. Reducing insulin resistance with metformin:the evidence today[J]. Diabetes Metab, 2003, 29(4 Pt 2): 6S28–6S35. |
| [11] | RENA G, PEARSON E R, SAKAMOTO K. Molecular mechanism of action of metformin:old or new insights?[J]. Diabetologia, 2013, 56: 1898–1906. DOI: 10.1007/s00125-013-2991-0 |
| [12] | ANDRADE B M, ARAUJO R L, PERRY R L, SOUZA E C, CAZARIN J M, CARVALHO D P, et al. A novel role for AMP-kinase in the regulation of the Na+/I--symporter and iodide uptake in the rat thyroid gland[J]. Am J Physiol Cell Physiol, 2011, 300: C1291–C1297. |
| [13] | ABDULRAHMAN R M, BOON M R, SIPS H C, GUIGAS B, RENSEN P C, SMIT J W, et al. Impact of metformin and compound C on NIS expression and iodine uptake in vitro and in vivo:a role for CRE in AMPK modulation of thyroid function[J]. Thyroid, 2014, 24: 78–87. DOI: 10.1089/thy.2013.0041 |
| [14] | VIGERSKY R A, FILMORE-NASSAR A, GLASS A R. Thyrotropin suppression by metformin[J]. J Clin Endocrinol Metab, 2006, 91: 225–227. DOI: 10.1210/jc.2005-1210 |
| [15] | LUPOLI R, DI M A, TORTORA A, AMBROSINO P, LUPOLI G A, DI M M. Effects of treatment with metformin on TSH levels:a meta-analysis of literature studies[J]. J Clin Endocrinol Metab, 2014, 99: 143–148. DOI: 10.1210/jc.2013-2965 |
| [16] | CARNEIRO I, CASTRO-PIEDRAS I, MUÑOZ A, LABANDEIRA-GARCÍA J L, DEVESA J, ARCE V M. Hypothyroidism is associated with increased myostatin expression in rats[J]. J Endocrinol Invest, 2008, 31: 773–778. DOI: 10.1007/BF03349256 |
| [17] | LÓPEZ M, VARELA L, VÁZQUEZ M J, RODRÍGUEZ-CUENCA S, GONZÁLEZ C R, VELAGAPUDI V R, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance[J]. Nat Med, 2010, 16: 1001–1008. DOI: 10.1038/nm.2207 |
| [18] | DOHÁN O, DE L V A, PARODER V, RIEDEL C, ARTANI M, REED M, et al. The sodium/iodide symporter (NIS):characterization, regulation, and medical significance[J]. Endocr Rev, 2003, 24: 48–77. DOI: 10.1210/er.2001-0029 |
| [19] | AEL-A H, ABBAS A M, EL WAKIL G A, ELSAMANOUDY A Z, EL WAKIL A A. Effect of chronic excess iodine intake on thyroid function and oxidative stress in hypothyroid rats[J]. Can J Physiol Pharmacol, 2012, 90: 617–625. DOI: 10.1139/y2012-046 |
| [20] | TAKI K, KOGAI T, KANAMOTO Y, HERSHMAN J M, BRENT G A. A thyroid-specific far-upstream enhancer in the human sodium/iodide symporter gene requires Pax-8 binding and cyclic adenosine 3', 5'-monophosphate response element-like sequence binding proteins for full activity and is differentially regulated in normal and thyroid cancer cells[J]. Mol Endocrinol, 2002, 16: 2266–2282. DOI: 10.1210/me.2002-0109 |
| [21] | SUE M, AKAMA T, KAWASHIMA A, NAKAMURA H, HARA T, TANIGAWA K, et al. Propylthiouracil increases sodium/iodide symporter gene expression and iodide uptake in rat thyroid cells in the absence of TSH[J]. Thyroid, 2012, 22: 844–852. DOI: 10.1089/thy.2011.0290 |
 2017, Vol. 38
2017, Vol. 38



