2. 第二军医大学东方肝胆外科医院病理科, 上海 200438
2. Department of Pathology, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai 200438, China
原发性肝癌 (hepatocellular carcinoma, HCC) 是临床最常见的恶性肿瘤之一,随着乙型病毒性肝炎和丙型病毒性肝炎的广泛传播、逐渐发展,其发生率逐年升高[1~2]。目前针对HCC的治疗方案主要有手术治疗、肝移植和经皮肝内治疗,且都具有较好的疗效,但肝切除术是目前国际公认的最适合的治疗手段[3]。肝功能是判断肝癌患者能否接受手术的主要因素,以往研究显示,行肝切除术的肝癌患者一般肝功能是Child-Pugh A级或是较早的B级[4]。但有些经过评估后适合手术的患者术后依然出现了严重的并发症甚至死亡,有些患者肿瘤复发时间及生存期较短[5~6]。前白蛋白 (prealbumin,PA) 是一种由肝细胞合成的血浆蛋白,只受肝细胞代谢影响,不因外源性补充而波动,在评估肝功能方面相对于其他指标如凝血酶原时间 (prothrombin time, PT)、白蛋白 (albumin,ALB) 更为可靠, 既往有研究认为PA含量是反映肝脏功能最适合的指标[7]。本研究拟探讨影响HCC患者肝切除术后远期预后的相关术前肝脏指标,并探讨术前血浆PA含量在评价患者远期预后中的价值。
1 资料和方法 1.1 病例选择本研究为回顾性研究。收集第二军医大学东方肝胆外科医院2011年12月—2012年3月560例接受肝切除术的HCC患者的病例资料及随访资料,严格安照制定的纳入标准和排除标准进行筛选。纳入标准:(1) 接受肝切除术的肝癌患者;(2) 切除标本经病理证实为HCC;(3) 预后随访资料及病例资料完整者。排除标准:(1) 合并其他原发性肿瘤的患者;(2) 病例或随访信息不完整者;(3) 死亡原因与原发肿瘤无关者;(4) 合并影响ALB及PA含量的其他疾病者。共373例纳入本研究,其中男性328例、女性45例,中位年龄52(25~81) 岁。临床病理和术前实验室资料见表 1。
|
|
表 1 患者基本临床病理特征 Tab 1 Basic characteristics of all patients |
1.2 资料收集
从患者病例资料中收集患者的性别、年龄、乙肝病毒 (hepatitis B virus, HBV) 感染史、吸烟史和饮酒史、Child-Pugh分级、术前实验室检测指标 (PA、ALB、TB、PT、AFP、CA19-9、CEA值) 及病理资料 (肿瘤数目、大小、病理形态、血管侵犯、有无肝硬化)。患者术前资料为患者第1次在我院住院尚未接受任何治疗之前获得。肿瘤的TNM分期结合患者术后病理资料,参照AJCC第7版TNM分期系统[8]得出。
1.3 临床指标临界值的界定我院临床使用数据中,判断PA含量高低的临界值为172 mg/L。为了验证临界值对数据准确性的影响程度,更好地明确PA含量对预后的影响,利用X-tile软件计算得出PA的最佳临界值,使相应组别患者生存期与复发状况比较时的P值最小。经过X-tile计算,PA含量以152 mg/L为临界值的P值为0.001 9,PA含量以172 mg/L为临界值的P值为0.089 8。说明PA含量152 mg/L为临界值比172 mg/L更有统计学意义。其他临床指标皆以临床临界值作为分组界值。
1.4 病例随访研究起始时间为接受肝切除术的术后第1天,结束时间为患者死亡、最后1次随访时间或失访时间。随访时间截至2016年12月1日,第1年患者每1个月进行1次随访,第2年每3个月接受1次随访,第3年及以后每6个月接受1次随访。随访内容包括患者术后有无复发、复发时间、生存状态、死亡时间、肝功能、肿瘤血清学指标 (AFP、CEA、CA19-9) 及影像学情况 (B超、CT、MRI)。若患者术后肿瘤指标明显增高且大于正常值,或影像学检测到新的病灶,则定义为肿瘤复发。随访期间若患者死于原发灶及相关疾病则患者为完全数据,若截至随访日期患者仍生存则按照截尾数据处理。
1.5 统计学处理所有数据采用Excel进行统计整理,以SPSS 22.0软件进行统计学分析。用单因素Cox回归分析得到与预后相关的因素,并用多因素Cox回归法得出独立预后因子。根据PA临界值152 mg/L将患者分为高PA组及低PA组,采用χ2检验比较两组之间分类资料的差异。以Kaplan-Meier曲线评估患者复发率及生存率,并用Log-rank方法检验两组间的差异。检验水准 (α) 为0.05。
2 结果 2.1 患者预后危险因素分析通过单因素分析发现,术前血清PA含量、肿瘤数量、肿瘤大小、TNM分期、肿瘤分化程度、微血管癌栓都是影响HCC患者术后生存的危险因素,肿瘤大小、肿瘤数量、术前血清PA含量、TNM分期、血管癌栓是影响HCC患者术后肿瘤复发的危险因素;进一步行多因素分析发现,肿瘤大小、TNM分期、PA含量、血管癌栓是影响HCC患者术后生存的独立危险因素,TNM分期、PA含量、血管侵犯是影响HCC患者术后肿瘤复发的独立危险因素 (表 2)。
|
|
表 2 患者生存状态和复发的单因素及多因素分析 Tab 2 Univariate and multivariate analysis of factors influencing overall survival and recurrence of patients |
2.2 PA与患者临床各数据之间的关系
根据PA临界值152 mg/L将患者分为高PA组及低PA组,比较2组临床病理特征的差异,结果如表 3所示。由表 3可见,低PA组中肿瘤直径>3 cm、PT>13 s、TB>34 μmol/L、ALB≤35 g/L的患者比例高于高PA组 (P < 0.05),且低PA组中TNM分期晚、肿瘤病理分期差、有血管癌栓的患者比例更高 (P < 0.05)。患者年龄、性别、肿瘤数量、AFP含量在两组间差异无统计学意义。
|
|
表 3 低PA组 (≤152 mg/L) 和高PA组 (>152 mg/L) 患者临床病理特征的比较 Tab 3 Comparison of clinicopatological characteristics of patients between low PA (≤152 mg/L) and high PA ( > 152 mg/L) groups |
2.3 PA与患者长期生存的关系
如图 1所示,低PA组HCC患者的中位生存时间为31.7个月,高PA组HCC患者的中位生存时间为41.3个月,高PA组比低PA组患者获得了更长的总生存时间 (P < 0.000 1);低PA组HCC患者肿瘤的中位复发时间为14.4个月,高PA组肝癌患者肿瘤的中位复发时间为28.8个月,高PA组比低PA组患者复发时间更长 (P < 0.000 1)。
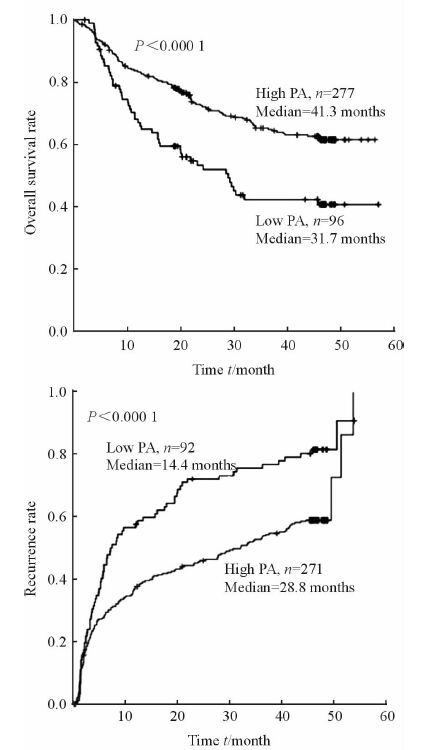
|
图 1 PA与患者生存时间和肿瘤复发时间的关系曲线 Fig 1 Correlation of preoperative prealbumin (PA) content with overall survival and recurrence time |
3 讨论
HCC是目前发病率及死亡率均居世界前列的恶性肿瘤[1, 5, 9]。目前,手术治疗依然是针对肝癌的最佳治疗方式,但患者术后肿瘤复发率高、长期生存情况较差。既往研究表明, 肝癌患者的AFP、肿瘤TNM分期及肝脏储备功能等因素可以用来预测肝癌患者术后肿瘤的复发风险及生存状态[10]。但这些指标的水平并不稳定,患者肿瘤堵塞胆管导致TB升高亦会导致结果波动, AFP水平在术前及术后阴性检测率高达30%以上[11~12], 而通过TNM分期对肝癌预后评估较为准确却存在滞后性,因此探究可靠的术前预测指标具有积极且重要的临床意义[10]。既往研究表明患者营养状态与术后并发症密切相关,而且也是影响术后肿瘤复发时间及长期生存状态的重要因素[13]。肿瘤患者的免疫抑制状态为癌细胞的发生、分裂及扩散提供了良好的微环境[14],外周血淋巴细胞是机体自身免疫的重要组成部分,越来越多的研究关注其在肿瘤研究中的潜在价值[15]。肿瘤患者的另一特征是机体功能的显著下降及营养状况不良,会导致自身合成ALB能力的下降,故而既往研究中常将ALB作为评估患者营养状态的重要指标[16]。但随着医疗技术的发展,更多的血浆制品被用于临床,患者通过外周血补充ALB的概率增加[17],加之ALB半衰期较长,约15~19 d,血浆浓度波动使得术前评估不准确;而且通过静脉补充ALB无法改善肿瘤患者的远期预后[18]。血浆PA是一种完全由肝细胞产生的载体蛋白,其主要生理功能是运输甲状腺素和维生素A,通过促进淋巴细胞成熟增强机体的免疫力[19]。研究表明,PA对肝细胞损伤具有极强的敏感性和特异性[20~21],因此,PA比ALB更适合作为评价肝癌患者营养状态、监测营养支持效果的指标。而且,在胃癌[22]、结肠癌[23]、肺癌[24]中的研究也表明PA与患者远期预后密切相关。
本研究通过预后危险因素分析,发现PA是影响肝癌肝切除术患者整体预后的独立危险因素之一。血清PA含量较低的HCC患者,其肿瘤复发时间较短,总生存期相对缩短。尽管PA影响肿瘤患者长期预后的具体机制尚未完全阐明,但免疫抑制在肿瘤的发生发展及复发中的作用已被广泛接受[25]。PA促进淋巴细胞的生成,其血清含量越低,淋巴细胞越少,进而造成机体低免疫状态[15]。由于PA完全由肝细胞产生,不受静脉补充干扰,具备评估肝细胞状态的能力,可以作为预测患者预后的潜在指标之一。
综上所述,根据本中心研究数据分析,我们认为PA与肝癌肝切除术患者的肿瘤复发及远期生存状态密切相关,可以作为辅助评估患者预后、选择治疗方案的潜在指标。由于本研究数据基于单中心,纳入病例数较少,其代表性存在一定的局限,这些因素可能会影响本研究结果的普遍性,因此还有待进一步的多中心大样本研究来验证。
| [1] | OMATA M, LESMANA L A, TATEISHI R, CHEN P J, LIN S M, YOSHIDA H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma[J]. Hepatol Int, 2010, 4: 439–474. DOI: 10.1007/s12072-010-9165-7 |
| [2] | FERENCI P, FRIED M, LABRECQUE D, BRUIX J, SHERMAN M, OMATA M, et al. Hepatocellular carcinoma (HCC):a global perspective[J]. J Clin Gastroenterol, 2010, 44: 239–245. DOI: 10.1097/MCG.0b013e3181d46ef2 |
| [3] | LURJE G, LESURTEL M, CLAVIEN P A. Multimodal treatment strategies in patients undergoing surgery for hepatocellular carcinoma[J]. Dig Dis, 2013, 31: 112–117. DOI: 10.1159/000347205 |
| [4] | FORNER A, LLOVET J M, BRUIX J. Hepatocellular carcinoma[J]. Lancet, 2012, 379: 1245–1255. DOI: 10.1016/S0140-6736(11)61347-0 |
| [5] | VILLANUEVA A, HOSHIDA Y, BATTISTON C, TOVAR V, SIA D, ALSINET C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma[J]. Gastroenterology, 2011, 140: 1501–1512. DOI: 10.1053/j.gastro.2011.02.006 |
| [6] | LLOVET J M, BRUIX J. Novel advancements in the management of hepatocellular carcinoma in 2008[J]. J Hepatol, 2008, 48: S20–S37. |
| [7] | MEARS E. Outcomes of continuous process improvement of a nutritional care program incorporating serum prealbumin measurements[J]. Nutrition, 1996, 12(7/8): 479–484. |
| [8] | EDGE S B, COMPTON C C. The American Joint Committee on Cancer:the 7th edition of the AJCC cancer staging manual and the future of TNM[J]. Ann Surg Oncol, 2010, 17: 1471–1474. DOI: 10.1245/s10434-010-0985-4 |
| [9] | YOUNOSSI Z M, OTGONSUREN M, HENRY L, VENKATESAN C, MISHRA A, ERARIO M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009[J]. Hepatology, 2015, 62: 1723–1730. DOI: 10.1002/hep.28123 |
| [10] | CAMMÀ C, CABIBBO G. Prognostic scores for hepatocellular carcinoma:none is the winner[J]. Liver Int, 2009, 29: 478–480. DOI: 10.1111/liv.2009.29.issue-4 |
| [11] | JOHNSON P J. The role of serum α-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma[J]. Clin Liver Dis, 2001, 5: 145–159. DOI: 10.1016/S1089-3261(05)70158-6 |
| [12] | MARKS R M, RYAN A, HEBA E R, TANG A, WOLFSON T J, GAMST A C, et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance[J]. AJR Am J Roentgenol, 2015, 204: 527–535. DOI: 10.2214/AJR.14.12986 |
| [13] | ESPER D H, HARB W A. The cancer cachexia syndrome:a review of metabolic and clinical manifestations[J]. Nutr Clin Pract, 2005, 20: 369–376. DOI: 10.1177/0115426505020004369 |
| [14] | RAY-COQUARD I, CROPET C, VAN GLABBEKE M, SEBBAN C, LE CESNE A, JUDSON I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas[J]. Cancer Res, 2009, 69: 5383–5391. DOI: 10.1158/0008-5472.CAN-08-3845 |
| [15] | DELHEM N, CARPENTIER A, MORALÉS O, MIROUX C, GROUX H, AURIAULT C, et al. [Regulatory T-cells and hepatocellular carcinoma:implication of the regulatory T lymphocytes in the control of the immune response][J]. Bull Cancer, 2008, 95: 1219–1225. |
| [16] | SUZANA S, BOON P C, CHAN P P, NORMAH C D. Malnutrition risk and its association with appetite, functional and psychosocial status among elderly Malays in an agricultural settlement[J]. Malays J Nutr, 2013, 19: 65–75. |
| [17] | HU L, XUE F, LI Y, SHAO M, SUN Y, WEI G. A long-term follow-up and comprehensive observation of risk and prognosis factors of recurrence and survival after resection of hepatocellular carcinoma[J]. Cell biochem Biophys, 2014, 69: 421–431. DOI: 10.1007/s12013-013-9813-3 |
| [18] | OHTA T, OGAWA K, NAGASE S. Elevation of serum albumin by intrahepatic transplantation of albumin-producing cells does not correct quantitative abnormalities of non-albumin proteins in analbuminemic rats[J]. Biochem Biophys Res Commu, 1993, 197: 1103–1110. DOI: 10.1006/bbrc.1993.2591 |
| [19] | ALIYAZICIOĞLU Y, DEĞER O, KARAHAN C, YILDIRMIŞ S, KÜÇÜKÖDÜK S. Reference values of cord blood transferrin, ceruloplasmin, α-1 antitrypsin, prealbumin, and α-2 macroglobulin concentrations in healthy term newborns[J]. Turk J Pediatr, 2007, 49: 52–54. |
| [20] | SAITO M, SEO Y, YANO Y, MIKI A, YOSHIDA M, AZUMA T. Short-term reductions in non-protein respiratory quotient and prealbumin can be associated with the long-term deterioration of liver function after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma[J]. J Gastroenterol, 2012, 47: 704–714. DOI: 10.1007/s00535-012-0535-x |
| [21] | LIU F, CAI L Y, ZHONG L, CHEN C, XU F, ZHAO Z X, et al. Model for end-stage liver disease combined with serum prealbumin to predict the prognosis of patients with decompensated liver cirrhosis[J]. J Dig Dis, 2010, 11: 352–357. DOI: 10.1111/cdd.2010.11.issue-6 |
| [22] | JIANG N, DENG J Y, DING X W, KE B, LIU N, ZHANG R P, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer[J]. World J Gastroenterol, 2014, 20: 10537–10544. DOI: 10.3748/wjg.v20.i30.10537 |
| [23] | TU M Y, CHIEN T W, CHOU M T. Using a nutritional screening tool to evaluate the nutritional status of patients with colorectal cancer[J]. Nutr Cancer, 2012, 64: 323–330. DOI: 10.1080/01635581.2012.650778 |
| [24] | KAWAI H, OTA H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer[J]. World J Surg, 2012, 36: 2853–2857. DOI: 10.1007/s00268-012-1766-y |
| [25] | QI Q, GENG Y, SUN M, WANG P, CHEN Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer[J]. Pancreatology, 2015, 15: 145–150. DOI: 10.1016/j.pan.2014.12.004 |
 2017, Vol. 38
2017, Vol. 38


