2. 中韩生物医学工程中心, 上海 201802
2. China-South Korea Biomedical Engineering Center, Shanghai 201802, China
骨质疏松症是一种常见的代谢性骨病,以骨量减少、骨密度降低和骨微结构破坏为特征,容易发生脆性骨折。骨质疏松症分为原发性和继发性两大类,原发性骨质疏松症又分为绝经后骨质疏松症、老年性骨质疏松症和特发性骨质疏松症 (包括青少年型)。绝经后骨质疏松症一般发生在女性绝经后5~10年内;老年性骨质疏松症一般指70岁后发生的骨质疏松;而特发性骨质疏松症主要发生在青少年,病因尚不明确。随着人口老龄化的加速,骨质疏松症的发病率逐年增高[1]。最新数据显示,我国骨质疏松症的患病人数已达1.4亿,女性多于男性,40岁以上人群骨质疏松症发病率为24.62%[2]。骨质疏松性骨折发病率不断增高,严重危害老龄人口的生活质量[3]。
骨质疏松症发病的根本原因在于骨吸收与骨形成的失衡。针对破骨细胞分化的研究已广泛且深入,多种抑制骨吸收的抗骨质疏松药物相继问世,如双膦酸盐类等。但在长期应用过程中人们发现,破骨细胞抑制剂抑制了骨改建过程,存在诸多不良反应,如下颌坏死等[4]。因此,如何促进骨形成近年来受到更多关注。
间充质干细胞 (mesenchymal stem cell, MSC) 是一类来源于中胚层的干细胞,具有多向分化潜能,存在于多种组织中,如肝、皮肤、胎盘等。其中骨髓来源的MSC (bone marrow-derived mesenchymal stem cell, BMSC) 研究最为广泛。BMSC是成骨细胞和脂肪细胞的前体干细胞,其分化去向在骨平衡中扮演了重要角色,其中成骨与成脂分化的调控是当前研究的热点[5]。研究发现BMSC向成骨分化减少、成脂分化增多是骨质疏松症发病的重要机制[6-8]。抑制BMSC成脂分化、促进其成骨分化从而纠正骨代谢失衡是治疗骨质疏松症的方向之一[9-11]。信号通路、microRNA (miRNA)、表观修饰等多种因素参与BMSC分化命运的调控,寻找决定BMSC分化方向的关键因子和MSC移植为骨质疏松症的治疗提供了新的思路。
1 生物因素:是否存在调控BMSC分化命运的关键因子?为了寻找决定BMSC分化方向的关键因子、探究骨质疏松治疗的新靶点,学者们从信号转导、基因转录及转录后水平开展了大量研究,信号通路调控、miRNA和表观修饰等因素是目前BMSC分化命运研究的热点与焦点。
1.1 调控BMSC分化的胞内信号转导通路多条信号通路参与了BMSC成骨、成脂分化的调控,包括骨形态发生蛋白 (bone morphogenic protein, BMP)/Smad通路、Wnt通路、Hedgehog通路、Notch通路和成纤维细胞生长因子 (fibroblast growth factor, FGF) 通路等 (图 1)。
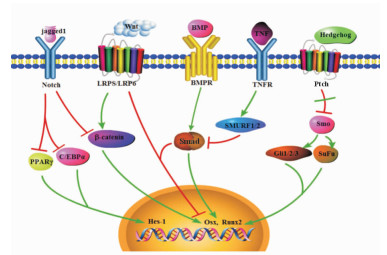
|
图 1 调控骨髓间充质干细胞分化的信号通路 Fig 1 Signal pathways regulated the differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) |
1.1.1 BMP/Smad通路
BMP属于转化生长因子 (transforming growth factor-β,TGFβ) 超家族,目前已发现30多个成员,它们在调控BMSC分化中具有不同功能,如BMP2能够促进成骨、成软骨分化,而BMP4单独能够促进MSC成脂分化[12]。Smad家族是BMP信号转导蛋白,包括Smad 1~9,根据功能可分为受体调节性Smad、中介型Smad和抑制型Smad。Smad激活后,与Co-Smads形成复合体,共同转移至细胞核内,通过其远端和近端的P2启动子启动Runx2基因的表达,而Runx2上的一段氨基酸区域正是成骨分化所必需[13]。BMP2通过Smad上调Msx2基因表达,而Msx2则可以通过Wnt通路促进成骨分化[14]。BMP7联合TGFβ1能够促进MSC向软骨细胞方向的分化[15]。BMP9通过促进Smad1和Smad5的磷酸化,活化p38而促进软骨形成[16]。BMP6能够显著增强碱性磷酸酶 (ALP)、Ⅰ型胶原α1链 (COL1A1) 和Osterix的表达,从而增强BMC的骨形成能力[17]。
1.1.2 Wnt通路Wnt家族由许多分泌型糖蛋白组成,与配体结合后能够稳定β-链蛋白 (β-catenin),阻止其磷酸化。未磷酸化的β-catenin能够转移至核内调控靶基因的转录,构成经典的Wnt通路。Wnt通路激活使得分泌的Wnt与细胞表面受体Fzd家族或LRPS/LRP6受体结合,激活Dsh蛋白。磷酸化的Dsh蛋白能够抑制APC、GSK-3D、Axin、β-catenin的激酶活性,最终引起β-catenin在细胞内的积累,从而激活下游基质金属蛋白酶3(MMP3) 等一系列基因,促进成骨分化,抑制成脂分化[18]。非经典Wnt通路在成骨分化中也起着重要作用。Wnt3a通过PP1A介导的去磷酸化激活TAZ刺激成骨分化[19]。YAP/TAZ是非经典Wnt通路的下游效应因子,可介导非经典Wnt通路的生物学功能,包括骨生成作用[20]。在小鼠体内过表达Wnt10b能够激活Wnt通路,促进成骨;相应的,缺乏Wnt10b则造成骨密度下降[21]。此外,研究表明年龄相关的脂肪增多与Wnt10b的减少有关[22]。Yu等[23]发现Wnt4通过非经典Wnt通路抑制巨噬细胞和破骨前体细胞中TGFβ激活激酶1介导的核因子κB (NF-κB),抑制破骨细胞形成和骨吸收,从而减缓绝经后骨质流失和年龄增长相关的骨质流失。
1.1.3 Notch通路Notch通路在MSC分化中扮演了双刃剑的角色。它既能抑制成骨分化,又为成骨分化所必需。在MSC中加入Notch配体jagged1或过表达Notch靶基因Hes-1后能够阻断PPARγ和C/EBPα的表达,抑制了成脂分化,促进成骨分化[24]。阻断Notch信号通路则可通过PTEN-PI3K/Akt/mTOR通路促进自噬介导的MSC成脂分化[25]。此外,Notch通路能够通过抑制Wnt/β-catenin通路进而抑制成骨分化[26]。Ongaro等[27]研究发现Notch通路参与人骨肉瘤细胞系MG63中成骨分化的发育阶段,并且对成骨分化有双重调节作用:激活Notch2、Notch4后能促进成骨分化,而激活Notch1、Notch3和Hes5后则使细胞保持在未分化状态。
1.1.4 Hedgehog通路Hedgehog信号通路的成分SHh、IHh、DHh和Gli 1~3在MSC中高表达,发挥正调节作用。在正常情况下,细胞膜上的Ptch与Smo结合并抑制Smo的活性,防止Smo激活下游通路。当Hedgehog与其受体结合后,其受体被激活,与Ptch结合,解除了对Smo的抑制。激活的Smo能够将信号向下游转导,激活Gli转录因子,包括Gli 1、2、3。随后Gli磷酸化并且启动细胞核内Ccdn1、Ccdn2、Foxa2、Wntx等基因表达,从而促进成骨分化[28]。在成脂分化中,Hedgehog信号下调。总体而言,Hedgehog通路激活促进了成骨,并与BMP信号有交叉作用[29]。
1.2 BMSC分化的转录水平调控参与MSC分化的转录因子是信号通路的直接或间接靶点,现已证实有多个转录因子参与MSC成骨、成脂分化。PPARγ和C/EBPα与MSC的成脂分化有关[6]。PPARγ是一种由配体激活的核转录因子,在脂肪组织大量表达,在血管壁细胞 (如单核/巨噬细胞、内皮细胞、平滑肌细胞) 及心肌细胞等也有表达[30]。PPARγ一方面在与配体结合后与其下游的p65、p50N亚基相结合直接抑制NF-κB的活性,另一方面通过抑制IκB的降解而抑制NF-κB的表达[31],从而在多种病理生理过程中起到重要的调节作用。C/EBPα属于CCAAT增强子结合蛋白家族,有2个亚型,较短蛋白为p30亚型,较长蛋白为p42亚型。C/EBPα通过对细胞内基因的转录进行正、负向调控,从而广泛参与生物体内的各种生理反应[32]。
Runx2和Osterix是成骨分化之必需[33]。Runx2主要有3型,其中Ⅲ型Runx2主要存在于小鼠骨组织和成骨细胞中。Kobayashi等[34]的研究表明,Runx2基因缺失的纯合子小鼠出生后由于没有形成肋骨、不能维持呼吸而很快死亡。此类小鼠四肢短小,完全缺乏骨化,其骨架由软骨组成,缺乏骨髓。当小鼠体内Runx2过度表达时,该小鼠表现出骨质减少伴多发骨折,在这些小鼠中成骨细胞多处于不成熟状态[35]。这两项研究表明Runx2决定着BMSC向成骨细胞分化,在早期促进成骨细胞的分化,而在晚期则抑制成骨细胞分化。Osterix主要表达在颅面骨成骨细胞、软骨细胞、骨肉瘤细胞及骨巨细胞瘤间质细胞中。过表达Osterix可通过抑制软骨细胞转录因子Sox9和Sox5而促使其向成骨细胞转化,Osterix还可阻止脂肪细胞特异性转录因子PPAR-C的表达而抑制脂肪细胞形成[36]。成骨细胞的最终分化成熟则需要Osterix的作用。
NF-κB可抑制MSC向成骨方向分化。Chang等[37]在2009年首次报道了NF-κB激活后能够抑制MSC细胞向成骨分化,并证明在已分化的成骨细胞中抑制NF-κB后能够显著减少卵巢切除小鼠骨质流失。炎症环境能够抑制MSC向成骨方向分化,TNF和IL-17能够激活MSC中的IKK-NF-κB通路,通过促进Smurf1和Smurf2表达促进β-catenin泛素化降解,抑制骨形成;而抑制IKK-NF-κB通路激活能够显著促进MSC介导的骨形成[38]。
1.3 BMSC分化的转录后调控miRNA是一类由内源基因编码的长度约为22个核苷酸的非编码单链RNA分子,它们参与转录后基因表达调控。近年来miRNA在BMSC成骨成脂分化中的调控作用引起人们关注,已有报道多个miRNA参与了BMSC的分化调控。miR-204作用于Runx2促进MSC成脂分化[7],miR-637抑制成骨分化、促进成脂分化[39]。miR-27b通过靶基因作用于PPARγ和C/EBPα的3′非翻译区域,从而抑制成脂分化、促进成骨分化[40]。miR-21是TGFβ通路的负调控因子,过表达miR-21能够解除TGFβ的抑制成脂效应[41]。Liao等[8, 42]的研究表明,TNF-α通过NF-κB途径抑制FoxO1而过表达miR-705和miR-3077-5p,从而调节HOXA10和Runx2转录而促进成脂分化。Li等[43]发现体内miR-188的表达随着年龄而增加,敲除小鼠中miR-188基因后能减缓小鼠中骨质流失和骨髓中脂肪堆积,进一步研究发现miR-188直接与HDAC9靶点结合从而促进BMSC成脂分化、抑制成骨分化。
除了调控成骨成脂平衡,有些miRNA对成骨分化和成脂分化有平行调控作用。过表达miR-335能够同时抑制成骨分化和成脂分化,进一步研究发现miR-335通过直接作用于Runx2调控MSC的分化[44]。相似的是,miR-138也能够同时抑制成骨分化和成脂分化[45]。
1.4 BMSC的表观修饰调节干细胞命运由转录因子与DNA的结合情况决定,这一过程在表观修饰层面得到精确调控。MSC分化依赖于相关基因表达的激活或抑制[46]。虽然表观修饰是MSC分化与胞外信号转导的重要机制,但目前对调控MSC成骨成脂分化的表观修饰仍然知之甚少。位于组蛋白氨基端赖氨酸的甲基化通过调控染色质的结构发挥重要作用。在干细胞分化过程中,表观遗传标记物表达水平的变化反映了基因的激活与抑制。越来越多的证据表明,组蛋白去甲基化酶与MSC分化有关[47]。BMP4/7异聚体能够通过Smad信号快速诱导KDM4B和KDM6B两种去甲基化酶的表达;敲除KDM4B和KDM6B降低了MSC的成骨能力,而增强了其成脂能力[48]。ALKBH1是最新发现的一种DNA N6甲基腺嘌呤去甲基化酶,在人MSC成骨分化过程中高表达;敲除ALKBH1后成骨相关基因表达受到抑制,缺失ALKBH1的MSC植入体内后成骨能力明显减弱[49]。在骨质疏松症患者中,通过芯片筛查后发现KDM5A表达显著升高,能够抑制BMP2的诱导成骨能力;动物实验证明KDM5A通过降低Runx2启动子区域中H3K4me3的水平调控MSC成骨分化[50]。
1.5 生物分子与骨质疏松症治疗BMSC在骨质疏松症发病中起到了重要作用,环境与自身因素的变化影响了BMSC的分化轨迹,其中涉及信号通路、miRNA、表观遗传修饰、lncRNA、外泌体等。最终的结果是BMSC向成骨方向分化减少,向脂肪方向分化增多,加剧了骨质的流失。据此理论,干预关键靶点、去除抑制成骨的因素是预防和治疗骨质疏松症的新思路。由于BMP-2在促进骨形成中起着关键作用,已被用于诱导BMSC的成骨分化,以BMP-2为靶点的药物筛选研究也有不少,但得到的药物往往缺乏骨组织特异性,生物利用度不高,仍有待进一步研究。Wang等[50]发现KDM5A抑制剂能够减少去卵巢小鼠骨量的丢失,认为该发现可为提高BMP2的骨质疏松症治疗作用提供新的策略。Montgomery等[28]发现了一种成骨细胞的氧化型胆固醇Oxy133,该小分子物质可诱导OSX、BSP和OCN的表达,并促进人BMSC的成骨作用,认为小分子成骨物质Oxy133有望作为一种新的骨合成代谢剂用于骨质疏松症的治疗。
总体而言,BMSC的分化是一个多步骤、多因素参与的过程,尚未发现特异性的分子或通路,对于MSC分化决定的关键步骤与关键因子仍然不清楚。目前尚无法找到特异的治疗靶点和药物,以及早进行干预,从根本上杜绝骨质疏松症的发生。
2 理化调控因素:BMSC是理疗的机制?电磁技术在骨科治疗中的应用已经有一个多世纪。脉冲电磁场刺激 (pulsed electromagnetic fields, PEMF) 于20世纪70年代被应用于临床,在骨质疏松症的治疗中也取得了一定的效果,然而其治疗机制尚不明了。动物实验表明,PEMF可提高骨质疏松症动物模型的骨密度,促进骨折的愈合并提高骨的生物力学性能[51-52]。Takayama等[53]研究显示PEMF (15 Hz) 可以显著增加去卵巢大鼠骨的骨量,在测量钙和其他骨矿物质的含量后,发现PEMF可以显著减少骨质流失。有研究从成骨分化的角度探讨PEMF的机制,发现低频PEMF可以促进成骨细胞分化[54-55]。Simmons等[56]发现在用PEMF (12 Hz,1.1 mT) 刺激后,MSC加速分化为成骨细胞。PEMF可以在增强人类BMSC成骨分化能力的同时促进骨修复[57-58]。
由此可见,促进BMSC的成骨分化是PEMF治疗骨质疏松症的机制之一。通过对PEMF机制的更多了解,其临床应用价值将变得更加显著,也有助于开发更多新的医用物理技术以提高骨质疏松症的疗效。
3 MSC移植:离临床应用还有多远?骨质疏松症的病理特点表现为成骨细胞数量和功能的下降,绝经、衰老后BMSC数量及活性都大大降低,因而理论上我们可以通过MSC移植来增加成骨分化治疗骨质疏松症。动物研究发现,自体或异体MSC移植能够增加骨质疏松症模型动物的骨量,可直接将BMSC注射至骨小梁表面[59]。BMSC能够参与受损的和病变的组织修复。Ocarino Nde等[60]报道,将从健康大鼠中分离得到的BMSC注射至骨质疏松大鼠股骨,结果显示治疗2个月后骨质疏松组大鼠股骨骨小梁数量与健康大鼠无异。Wang等[61]将BMSC与藻酸钙凝胶复合后注射入骨质疏松的兔股骨远端,结果显示骨形成增多,骨密度增加。一项纳入了12项临床前动物研究的meta分析考察了MSC移植对于骨质疏松动物骨密度的影响,发现MSC移植能够改善动物骨质疏松[62]。
MSC移植具有诸多优点,如免疫排斥轻、来源广泛等,但骨质疏松症是一种全身性疾病,患者体内激素分泌水平、局部微环境中的细胞因子水平都发生了很大变化,MSC移植能否改善这些变化还需要进一步研究。此外,MSC移植尚面临诸多难题:(1) 体内安全性有待评估。体外扩增的MSC移植后在体内长期存在是否有致瘤风险目前仍未可知。(2) MSC体外活性的维持。体外培养条件下如何更好地维持MSC的干性目前尚无确切答案。(3) MSC移植后迁移与分化。如何促进移植的MSC在体内的分化率和存活率并向目标区域迁移,如何把握MSC移植的合适时间和数量,如何促进MSC向成骨细胞的分化,也是亟待解决的问题。(4) MSC来源。MSC有多个来源,包括骨髓、牙髓、脐带、胎盘、脂肪等[63]。目前研究中用于成骨治疗的MSC主要为骨髓来源的MSC (即BMSC),但面临取材困难、存在伦理障碍等难题。
4 小结和展望在骨质疏松症的发病机制和治疗手段的研究中,BMSC是近几年来的热点和焦点。现有研究表明:BMSC的分化是一个复杂事件,受胞外信号、理化因素、胞内信号转导、基因表观修饰、转录、转录后调控等多层次、多因素的影响,其分化走向是多因素共同作用的结果,但在限定的时间和空间内关键因素起主导作用。目前尚未发现决定MSC分化的特异性的分子或通路。理论上MSC移植为骨质疏松症提供了根本的治疗方法,但目前只在动物实验中取得了一定效果。基于此,我们认为未来的研究主要集中在几下以个方面:(1) BMSC在骨质疏松症发病中的角色和影响其分化的关键事件,即寻找到骨质疏松症的关键基因、调控分子等。未来研究方向即要揭示在骨质疏松症发病过程中BMSC分化的不同阶段中决定其分化走向的关键调控因素,精准调控其分化走向,实现骨质疏松症的预防和治疗。(2) MSC治疗骨质疏松症的有效性及安全性评价。相较BMSC,脂肪来源的MSC是近年来的热点,其具备成骨分化能力,具有受年龄影响较小、取材方便、来源广泛等优点,有潜力成为治疗细胞。我们近年对脂肪MSC展开了相关研究,期望能实现临床转化。无论采用哪种MSC,其有效性和安全性都需要深入研究。(3) MSC来源外泌体的研究和应用。MSC来源外泌体中包含miRNA、调控因子等成分,极有可能参与了BMSC间的信号传递并影响其分化走向。寻找外泌体中关键调控因素有望替代MSC用于疾病的治疗[64]。
| [1] | COLE Z A, DENNISON E M, COOPER C. Osteoporosis epidemiology update[J]. Curr Rheumatol Rep, 2008, 10: 92–96. DOI: 10.1007/s11926-008-0017-6 |
| [2] | 张智海, 张智若, 刘忠厚, 袁伟. 中国大陆地区以-2.0 SD为诊断标准的骨质疏松症发病率回顾性研究[J]. 中国骨质疏松杂志, 2016, 22: 1–8. DOI: 10.3969/j.issn.1006-7108.2016.01.001 |
| [3] | XIA W B, HE S L, XU L, LIU A M, JIANG Y, LI M, et al. Rapidly increasing rates of hip fracture in Beijing, China[J]. J Bone Miner Res, 2012, 27: 125–129. DOI: 10.1002/jbmr.519 |
| [4] | TURNER B, DRUDGE-COATES L, ALI S, PATI J, NARGUND V, ALI E, et al. Osteonecrosis of the jaw in patients receiving bone-targeted therapies: an overview--part Ⅰ[J]. Urol Nurs, 2016, 36: 111–116, 154. |
| [5] | CHEN Q, SHOU P, ZHENG C, JIANG M, CAO G, YANG Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?[J]. Cell Death Differ, 2016, 23: 1128–1139. DOI: 10.1038/cdd.2015.168 |
| [6] | KIM J, KO J. A novel PPARγ2 modulator sLZIP controls the balance between adipogenesis and osteogenesis during mesenchymal stem cell differentiation[J]. Cell Death Differ, 2014, 21: 1642–1655. DOI: 10.1038/cdd.2014.80 |
| [7] | HUANG J, ZHAO L, XING L, CHEN D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation[J]. Stem Cells, 2010, 28: 357–364. |
| [8] | LIAO L, YANG X, SU X, HU C, ZHU X, YANG N, et al. Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow[J]. Cell Death Dis, 2013, 4: e600. DOI: 10.1038/cddis.2013.130 |
| [9] | KIM M, LEE Y J, JEE S C, CHOI I, SUNG J S. Anti-adipogenic effects of sesamol on human mesenchymal stem cells[J]. Biochem Biophys Res Commun, 2016, 469: 49–54. DOI: 10.1016/j.bbrc.2015.11.070 |
| [10] | AN Q, WU D, MA Y, ZHOU B, LIU Q. Suppression of Evi1 promotes the osteogenic differentiation and inhibits the adipogenic differentiation of bone marrow-derived mesenchymal stem cells in vitro[J]. Int J Mol Med, 2015, 36: 1615–1622. |
| [11] | WANG C, MENG H, WANG X, ZHAO C, PENG J, WANG Y. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis[J]. Med Sci Monit, 2016, 22: 226–233. DOI: 10.12659/MSM.897044 |
| [12] | TANG Q Q, OTTO T C, LANE M D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage[J]. Proc Natl Acad Sci USA, 2004, 101: 9607–9611. DOI: 10.1073/pnas.0403100101 |
| [13] | DENG Z L, SHARFF K A, TANG N, SONG W X, LUO J, LUO X, et al. Regulation of osteogenic differentiation during skeletal development[J]. Front Biosci, 2008, 13: 2001–2021. DOI: 10.2741/2819 |
| [14] | ZHAI Y, IURA A, YEASMIN S, WIESE A B, WU R, FENG Y, et al. MSX2 is an oncogenic downstream target of activated WNT signaling in ovarian endometrioid adenocarcinoma[J]. Oncogene, 2011, 30: 4152–4162. DOI: 10.1038/onc.2011.123 |
| [15] | LEE P T, LI W J. Chondrogenesis of embryonic stem cell-derived mesenchymal stem cells induced by TGFβ1 and BMP7 Through increased TGFβ receptor expression and endogenous TGFβ1 production[J]. J Cell Biochem, 2017, 118: 172–181. DOI: 10.1002/jcb.v118.1 |
| [16] | CHENG A, GUSTAFSON A R, SCHANER TOOLEY C E, ZHANG M. BMP-9 dependent pathways required for the chondrogenic differentiation of pluripotent stem cells[J]. Differentiation, 2016, 92: 298–305. DOI: 10.1016/j.diff.2016.03.005 |
| [17] | MADHU V, LI C J, DIGHE A S, BALIAN G, CUI Q. BMP-non-responsive Sca1+ CD73+ CD44+ mouse bone marrow derived osteoprogenitor cells respond to combination of VEGF and BMP-6 to display enhanced osteoblastic differentiation and ectopic bone formation[J]. PLoS One, 2014, 9: e103060. DOI: 10.1371/journal.pone.0103060 |
| [18] | JACOBSEN C M, SCHWARTZ M A, ROBERTS H J, LIM K E, SPEVAK L, BOSKEY A L, et al. Enhanced Wnt signaling improves bone mass and strength, but not brittleness, in the Col1a1(+/mov13) mouse model of type Ⅰ osteogenesis imperfecta[J]. Bone, 2016, 90: 127–132. DOI: 10.1016/j.bone.2016.06.005 |
| [19] | BYUN M R, HWANG J H, KIM A R, KIM K M, HWANG E S, YAFFE M B, et al. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation[J]. Cell Death Differ, 2014, 21: 854–863. DOI: 10.1038/cdd.2014.8 |
| [20] | PARK H W, KIM Y C, YU B, MOROISHI T, MO J S, PLOUFFE S W, et al. Alternative Wnt signaling activates YAP/TAZ[J]. Cell, 2015, 162: 780–794. DOI: 10.1016/j.cell.2015.07.013 |
| [21] | BENNETT C N, OUYANG H, MA Y L, ZENG Q, GERIN I, SOUSA K M, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation[J]. J Bone Miner Res, 2007, 22: 1924–1932. DOI: 10.1359/jbmr.070810 |
| [22] | KUEM N, SONG S J, YU R, YUN J W, PARK T. Oleuropein attenuates visceral adiposity in high-fat diet-induced obese mice through the modulation of WNT10b-and galanin-mediated signalings[J]. Mol Nutr Food Res, 2014, 58: 2166–2176. DOI: 10.1002/mnfr.v58.11 |
| [23] | YU B, CHANG J, LIU Y, LI J, KEVORK K, AL-HEZAIMI K, et al. Wnt4 signaling prevents skeletal aging and inflammation by inhibiting nuclear factor-κB[J]. Nat Med, 2014, 20: 1009–1017. DOI: 10.1038/nm.3586 |
| [24] | RAMASAMY S K, KUSUMBE A P, WANG L, ADAMS R H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone[J]. Nature, 2014, 507: 376–380. DOI: 10.1038/nature13146 |
| [25] | SONG B Q, CHI Y, LI X, DU W J, HAN Z B, TIAN J J, et al. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway[J]. Cell Physiol Biochem, 2015, 36: 1991–2002. DOI: 10.1159/000430167 |
| [26] | LIN G L, HANKENSON K D. Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation[J]. J Cell Biochem, 2011, 112: 3491–3501. DOI: 10.1002/jcb.v112.12 |
| [27] | ONGARO A, PELLATI A, BAGHERI L, RIZZO P, CALICETI C, MASSARI L, et al. Characterization of Notch signaling during osteogenic differentiation in human osteosarcoma cell line MG63[J]. J Cell Physiol, 2016, 231: 2652–2663. DOI: 10.1002/jcp.25366 |
| [28] | MONTGOMERY S R, NARGIZYAN T, MELITON V, NACHTERGAELE S, ROHATGI R, STAPPENBECK F, et al. A novel osteogenic oxysterol compound for therapeutic development to promote bone growth: activation of hedgehog signaling and osteogenesis through smoothened binding[J]. J Bone Miner Res, 2014, 29: 1872–1885. DOI: 10.1002/jbmr.2213 |
| [29] | LI L, DONG Q, WANG Y, FENG Q, ZHOU P, OU X, et al. Hedgehog signaling is involved in the BMP9-induced osteogenic differentiation of mesenchymal stem cells[J]. Int J Mol Med, 2015, 35: 1641–1650. |
| [30] | GANSS R. Maternal metabolism and vascular adaptation in pregnancy: the PPAR link[J]. Trends Endocrinol Metab, 2017, 28: 73–84. DOI: 10.1016/j.tem.2016.09.004 |
| [31] | GRABACKA M, PIERZCHALSKA M, DEAN M, REISS K. Regulation of ketone body metabolism and the role of PPAR alpha[J]. Int J Mol Sci, 2016, 17: 17: 2093. https://en.wikipedia.org/wiki/Peroxisome_proliferator-activated_receptor_alpha |
| [32] | PULIDO-SALGADO M, VIDAL-TABOADA J M, SAURA J. C/EBPβ and C/EBPδ transcription factors: Basic biology and roles in the CNS[J]. Prog Neurobiol, 2015, 132: 1–33. DOI: 10.1016/j.pneurobio.2015.06.003 |
| [33] | LEE J S, LEE J M, IM G I. Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells[J]. Biomaterials, 2011, 32: 760–768. DOI: 10.1016/j.biomaterials.2010.09.042 |
| [34] | KOBAYASHI H, GAO Yh, UETA C, YAMAGUCHI A, KOMORI T. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro[J]. Biochem Biophys Res Commun, 2000, 273: 630–636. DOI: 10.1006/bbrc.2000.2981 |
| [35] | LIU W, TOYOSAWA S, FURUICHI T, KANATANI N, YOSHIDA C, LIU Y, et al. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures[J]. J Cell Biol, 2001, 155: 157–166. DOI: 10.1083/jcb.200105052 |
| [36] | LEE J E, KIM M H, HONG J, CHOI H J, PARK J, YANG W M. Effects of Osteo-F, a new herbal formula, on osteoporosis via up-regulation of Runx2 and Osterix[J]. Rsc Adv, 2017, 7: 1032–1037. DOI: 10.1039/C6RA25236B |
| [37] | CHANG J, WANG Z, TANG E, FAN Z, MCCAULEY L, FRANCESCHI R, et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB[J]. Nat Med, 2009, 15: 682–689. DOI: 10.1038/nm.1954 |
| [38] | CHANG J, LIU F, LEE M, WU B, TING K, ZARA J N, et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation[J]. Proc Natl Acad Sci USA, 2013, 110: 9469–9474. DOI: 10.1073/pnas.1300532110 |
| [39] | ZHANG J F, FU W M, HE M L, WANG H, WANG W M, YU S C, et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix[J]. Mol Biol Cell, 2011, 22: 3955–3961. DOI: 10.1091/mbc.E11-04-0356 |
| [40] | SONG C, YAO J, CAO C, LIANG X, HUANG J, HAN Z, et al. PPARγ is regulated by miR-27b-3p negatively and plays an important role in porcine oocyte maturation[J]. Biochem Biophys Res Commun, 2016, 479: 224–230. DOI: 10.1016/j.bbrc.2016.09.046 |
| [41] | CHOI S I, JIN J Y, MAENG Y S, KIM T I, KIM E K. TGF-β regulates TGFBIp expression in corneal fibroblasts via miR-21, miR-181a, and Smad signaling[J]. Biochem Biophys Res Commun, 2016, 472: 150–155. DOI: 10.1016/j.bbrc.2016.02.086 |
| [42] | LIAO L, SU X, YANG X, HU C, LI B, LV Y, et al. TNF-α inhibits FoxO1 by upregulating miR-705 to aggravate oxidative damage in bone marrow-derived mesenchymal stem cells during osteoporosis[J]. Stem Cells, 2016, 34: 1054–1067. DOI: 10.1002/stem.v34.4 |
| [43] | LI C J, CHENG P, LIANG M K, CHEN Y S, LU Q, WANG J Y, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation[J]. J Clin Invest, 2015, 125: 1509–1522. DOI: 10.1172/JCI77716 |
| [44] | TOMÉ M, LÓPEZ-ROMERO P, ALBO C, SEPÚ LVEDA J C, FERNÁNDEZ-GUTIÉRREZ B, DOPAZO A, et al. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells[J]. Cell Death Differ, 2011, 18: 985–995. DOI: 10.1038/cdd.2010.167 |
| [45] | ESKILDSEN T, TAIPALEENMÄKI H, STENVANG J, ABDALLAH B M, DITZEL N, NOSSENT A Y, et al. MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo[J]. Proc Natl Acad Sci USA, 2011, 108: 6139–6144. DOI: 10.1073/pnas.1016758108 |
| [46] | TYNDALL A, VAN LAAR J M. Stem cell transplantation and mesenchymal cells to treat autoimmune diseases[J]. Presse Med, 2016, 45(6 Pt 2): e159–e169. |
| [47] | AGGER K, CLOOS P A, RUDKJAER L, WILLIAMS K, ANDERSEN G, CHRISTENSEN J, et al. The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene-and stress-induced senescence[J]. Genes Dev, 2009, 23: 1171–1176. DOI: 10.1101/gad.510809 |
| [48] | YE L, FAN Z, YU B, CHANG J, AL HEZAIMI K, ZHOU X, et al. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs[J]. Cell Stem Cell, 2012, 11: 50–61. DOI: 10.1016/j.stem.2012.04.009 |
| [49] | ZHOU C, LIU Y, LI X, ZOU J, ZOU S. DNA N6-methyladenine demethylase ALKBH1 enhances osteogenic differentiation of human MSCs[J]. Bone Res, 2016, 4: 16033. DOI: 10.1038/boneres.2016.33 |
| [50] | WANG C, WANG J, LI J, HU G, SHAN S, LI Q, et al. KDM5A controls bone morphogenic protein 2-induced osteogenic differentiation of bone mesenchymal stem cells during osteoporosis[J]. Cell Death Dis, 2016, 7: e2335. DOI: 10.1038/cddis.2016.238 |
| [51] | SHEN W W, ZHAO J H. Pulsed electromagnetic fields stimulation affects BMD and local factor production of rats with disuse osteoporosis[J]. Bioelectromagnetics, 2010, 31: 113–119. |
| [52] | ANDROJNA C, FORT B, ZBOROWSKI M, MIDURA R J. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture[J]. Bioelectromagnetics, 2014, 35: 396–405. DOI: 10.1002/bem.v35.6 |
| [53] | TAKAYAMA K, NOMURA H, TANAKA J, ZBOROWSKI M, HARASAKI H, JACOBS G B, et al. Effect of a pulsing electromagnetic field on metabolically derived osteoporosis in rats: a pilot study[J]. ASAIO Trans, 1990, 36: M426–M428. |
| [54] | TSAI M T, LI W J, TUAN R S, CHANG W H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation[J]. J Orthop Res, 2009, 27: 1169–1174. DOI: 10.1002/jor.v27:9 |
| [55] | ONGARO A, PELLATI A, BAGHERI L, FORTINI C, SETTI S, DE MATTEI M. Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells[J]. Bioelectromagnetics, 2014, 35: 426–436. DOI: 10.1002/bem.v35.6 |
| [56] | SIMMONS Jr J W, MOONEY V, THACKER I. Pseudarthrosis after lumbar spine fusion: nonoperative salvage with pulsed electromagnetic fields[J]. Am J Orthop (Belle Mead NJ), 2004, 33: 27–30. |
| [57] | FU Y C, LIN C C, CHANG J K, CHEN C H, TAI I C, WANG G J, et al. A novel single pulsed electromagnetic field stimulates osteogenesis of bone marrow mesenchymal stem cells and bone repair[J]. PLoS One, 2014, 9: e91581. DOI: 10.1371/journal.pone.0091581 |
| [58] | SUN L Y, HSIEH D K, LIN P C, CHIU H T, CHIOU T W. Pulsed electromagnetic fields accelerate proliferation and osteogenic gene expression in human bone marrow mesenchymal stem cells during osteogenic differentiation[J]. Bioelectromagnetics, 2010, 31: 209–219. |
| [59] | ICHIOKA N, INABA M, KUSHIDA T, ESUMI T, TAKAHARA K, INABA K, et al. Prevention of senile osteoporosis in SAMP6 mice by intrabone marrow injection of allogeneic bone marrow cells[J]. Stem Cells, 2002, 20: 542–551. DOI: 10.1634/stemcells.20-6-542 |
| [60] | OCARINO NDE M, BOELONI J N, JORGETTI V, GOMES D A, GOES A M, SERAKIDES R. Intra-bone marrow injection of mesenchymal stem cells improves the femur bone mass of osteoporotic female rats[J]. Connect Tissue Res, 2010, 51: 426–433. DOI: 10.3109/03008201003597049 |
| [61] | WANG Z, GOH J, DAS DE S, GE Z, OUYANG H, CHONG J S, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model[J]. Tissue Eng, 2006, 12: 1753–1761. DOI: 10.1089/ten.2006.12.1753 |
| [62] | LI F, ZHOU C, XU L, TAO S, ZHAO J, GU Q. Effect of stem cell therapy on bone mineral density: a meta-analysis of preclinical studies in animal models of osteoporosis[J]. PLoS One, 2016, 11: e0149400. DOI: 10.1371/journal.pone.0149400 |
| [63] | SZEPESI Á, MATULA Z, SZIGETI A, VÁRADY G, SZALMA J, SZABÓ G, et al. In vitro characterization of human mesenchymal stem cells isolated from different tissues with a potential to promote complex bone regeneration[J]. Stem Cells Int, 2016, 2016: 3595941. DOI: 10.1155/2016/3595941 |
| [64] | PASHOUTAN SARVAR D, SHAMSASENJAN K, AKBARZADEHLALEH P. Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy[J]. Adv Pharm Bull, 2016, 6: 293–299. DOI: 10.15171/apb.2016.041 |
 2017, Vol. 38
2017, Vol. 38


