2. 上海体育学院运动科学学院运动生理化学与营养学教研室, 上海 200438;
3. 第二军医大学热带医学与公共卫生学系生物防御教研室, 上海市医学生物防护重点实验室, 上海 200433
2. Department of Sports Physiology and Chemistry, College of Sports Science, Shanghai University of Sports, Shanghai 200438, China;
3. Department of Biological Defense (Microbiology), Shanghai Key Laboratory of Medical Biodefence, Faculty of Tropical Medicine and Public Health, Second Military Medical University, Shanghai 200433, China
热环境可分为湿热环境和干热环境。我国长江以南的大部分地区为湿热环境,夏季温度超过35 ℃,甚至40 ℃的极端高温天气并不罕见,并且昼夜温差小,湿度高。这种高湿热环境严重影响人体散热,而在此环境下从事高强度体力活动时,产热增加和散热困难更易导致体温升高,热应激水平加剧,严重者可致热应激损伤、中暑甚至死亡[1~2]。由于中枢神经系统特别是大脑皮质的神经元对热刺激非常敏感,而心脏也是一个对温度敏感的器官, 当体温轻度升高后,交感神经兴奋性增强,心率加快,心肌收缩力增强,心输出量和心肌代谢率增加,心肌酶活性也增强[3~5];但当体温进一步升高后则易发生中暑现象, 可引起神经元水肿变性,机体心动过速、心力衰竭、心腔扩大、心肌过度拉伸,进而导致血氧饱和度降低、组织器官能量利用障碍[6]。
线粒体为各种细胞的供能中心,同时也是活性氧 (reactive oxygen species,ROS) 等氧自由基的产生部位。体温升高作为一种应激刺激源,早期使线粒体氧化呼吸链的各种酶活性升高,产能增加;但当体温进一步增加后,则引发酶活性降低、自由基产生增加,氧化呼吸链传递电子和氢离子效率下降,细胞内有机酸堆积,加重组织细胞的代谢障碍,甚至线粒体膜损伤、酶和氧自由基的外泄,最终导致整个细胞的水肿变性甚至溶解坏死[7]。因此,如何保持大脑皮质和心肌线粒体结构和功能的正常,对于改善高温条件下能量的正常产生和利用,预防热应激损伤甚至中暑的发生均具有极其重要的意义。
热习服是提高机体对湿热环境耐受能力的重要措施之一,而在高温条件下适度进行中低强度的体力活动可明显缩短习服的时间,如大鼠在高温条件下自由活动约28 d可获得习服[8],但在高温条件下每天中强度 (14 m/min) 运动1 h,14 d即可获得习服,表现为对湿热条件下高强度运动的耐受力明显增强,死亡率明显降低[9]。Tamura等[10]研究证明,热习服后骨骼肌线粒体的适应性增强,但是有关经过不同条件热习服训练后的大鼠在湿热条件下高强度运动刺激后大脑皮质和心肌线粒体形态及功能变化的研究较少。本研究以不同条件热习服训练后的大鼠为研究对象,观察其在湿热环境下进行高强度运动后血浆中应激相关激素的变化,在此基础上重点观察热习服对大鼠大脑皮质和心肌线粒体超微结构、氧化应激产物、能量代谢相关酶活性的影响,以阐明热习服训练对湿热环境下高强度运动大鼠线粒体功能的保护机制。
1 材料和方法 1.1 实验动物及分组雄性SD大鼠40只[由第二军医大学实验动物中心提供,动物生产许可证号:SCXK (沪)2013-0016],初始体质量 (160±10) g,分笼饲养,每笼5只,自由摄取食物和水。环境温度 (24±1) ℃,相对湿度50%~60%,采取12 h:12 h循环照明 (8:00~20:00光照)。适应性饲养3 d后,通过负重游泳力竭实验剔除游泳时间过短和过长的大鼠各4只,以保证参试大鼠体力活动能力的均衡性。其余32只大鼠随机分为常温自由活动 (room-temperature activity, RA) 组、常温运动 (room-temperature exercise, RE) 组、高温自由活动 (high-temperature activity,HA) 组和高温运动 (high-temperature exercise,HE) 组,每组8只。
1.2 热习服训练模型的建立实验所用高温舱为我室自建的高温舱,实验期间舱内湿度保持在 (60±5)%、温度维持在 (24±1) ℃和 (32±1) ℃,分别构成常温常湿和高温常湿环境。各组大鼠测量体质量和肛温后,置于上述相应环境内连续进行热习服训练14 d (2 h/d)。其中,RA和HA组的大鼠在饲养笼内自由活动2 h/d,RE和HE组大鼠在饲养笼内自由活动1 h/d后,在动物跑步机 (上海奥尔科特生物有限公司) 上匀速跑步1 h/d (坡度为0°、速度为14 m/min),热习服训练过程中禁食水。
1.3 热环境中高强度运动刺激在室温下饲养2 d后 (热习服训练结束后的第3天),各组大鼠均于9:00~11:00在湿热环境下 (温度36 ℃、相对湿度85%) 接受中高强度的跑步运动 (坡度为0°,速度为21 m/min) 30 min。
1.4 物理指标的测量和样品采集连续14 d热习服训练结束后的第2天,在乙醚麻醉下对32只大鼠进行眼眶取血1.5 mL,盛入含肝素锂的离心管内,3 500×g离心10 min,取血浆储存于-80 ℃冰箱备用。热环境运动刺激前后,分别测量体质量和肛温,根据公式计算体质量丢失率和肛温变化值 (Δt):体质量丢失率 (%)=(运动刺激前体质量-运动刺激后体质量)/运动刺激前体质量×100%;Δt=运动刺激后肛温-运动刺激前肛温。测量体质量和肛温后,立即在10%水合氯醛 (250 mg/kg) 麻醉下腹主动脉取血5 mL,离心后取血浆放入-80 ℃冰箱备用。取血完毕后,每组随机选取6只大鼠用100 mL的4 ℃生理盐水经升主动脉灌流冲洗血液,然后快速切取部分左心室心尖部心肌和大脑顶叶皮质放入液氮内,移入-80 ℃冰箱备用;每组的另外2只大鼠用生理盐水灌注后,再用含1.25%戊二醛、4%多聚甲醛的0.1 mol/L磷酸盐缓冲液 (pH 7.4) 400 mL灌注固定,在体视显微镜下取左心室心尖部的心肌和顶叶皮质各约1 mm3,放入含4%多聚甲醛的磷酸盐缓冲液内,送至第二军医大学电镜室。标本经常规电镜包埋,超薄切片,醋酸铀和枸橼酸铅双重染色,采用透射电镜 (日立7650,上海冉超光电科技有限公司) 观察并照相。
1.5 应激相关激素的测量将湿热环境运动刺激前后的大鼠血浆 (8只/组) 送至第二军医大学神经生物学教研室,采用放射免疫方法分别测定去甲肾上腺素 (norepinephrine,NE)、皮质醇 (cortisol,Cort) 和血管加压素 (arginine vasopressin,AVP) 的含量,具体方法参见文献[11]。
1.6 线粒体三磷酸腺苷 (ATP) 合成酶、ROS及组织总抗氧化能力的测量将未经灌注固定的大鼠大脑皮质和心肌组织 (6只/组) 从-80 ℃冰箱中取出,采用差速离心法提取线粒体悬浮液[12]。取部分线粒体悬浮液,使用大鼠线粒体呼吸链复合物Ⅴ(F0F1-ATP酶/ATP合成酶) ELISA检测试剂盒 (上海逸峰ELISA试剂盒生物公司) 按说明书进行检测,经酶标仪 (上海赛默飞世尔) 在450 nm波长下测定光密度 (D) 值,参考蛋白标准曲线计算大脑皮质和心肌线粒体中ATP合成酶的含量。另取部分线粒体悬浮液,使用大鼠ROS ELISA检测试剂盒 (上海慧颖生物技术有限公司),在450 nm波长下测定D值,计算大脑皮质和心肌线粒体中ROS的含量。另取部分大鼠大脑皮质和心肌组织,每20 mg组织加入100 μL冰冷的PBS匀浆以充分破碎组织并释放其中的抗氧化物,在4 ℃下、3 500×g离心10 min后取上清液,使用总抗氧化能力检测试剂盒 (ABTS法,碧云天生物技术研究所),在725~745 nm波长下测定D值,参考标准曲线计算大脑皮质和心肌组织总抗氧化能力。
1.7 统计学处理所有实验数据使用SPSS 21.0软件进行分析,采用GraphPad Prism 5.0软件绘图。数据结果以x±s表示,组内比较采用配对t检验,组间比较采用多个样本均数多重比较的Dunnett-t检验。检验水准 (α) 为0.05。
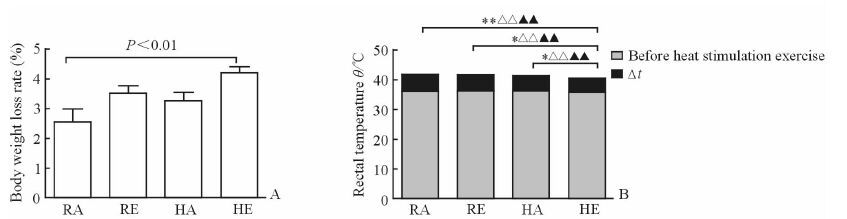
2 结果 2.1 湿热环境运动刺激对大鼠体质量丢失率和肛温的影响各组大鼠均接受高温高湿中高强度运动刺激30 min后,HE组大鼠的体质量丢失率大于RA组 (P<0.01),而HE组与RE、HA组间差异均无统计学意义 (图 1A)。如图 1B所示,HE组大鼠运动刺激前、后的肛温均低于RA组、RE组和HA组 (P<0.01),HE组大鼠的Δt低于RA组、RE组和HA组 (P<0.05,P<0.01)。
|
图 1 湿热环境下运动刺激前、后大鼠体质量丢失率和肛温变化 Fig 1 Body weight loss rates and rectal temperatures of rats before and after stimulation with exercise in hygrothermal environment A: Body weight loss rate; B: Rectal temperature. RA: Room-temperature activity; RE: Room-temperature exercise; HA: High-temperature activity; HE: High-temperature exercise. Δt=Rectal temperature after heat stimulation exercise-rectal temperature before heat stimulation exercise. *P < 0.05, **P < 0.01 in Δt; △△P < 0.01 in rectal temperature before heat stimulation exercise; ▲▲P < 0.01 in rectal temperature after heat stimulation exercise. n=8, x±s |
2.2 湿热环境运动刺激前后各组大鼠应激相关激素的变化
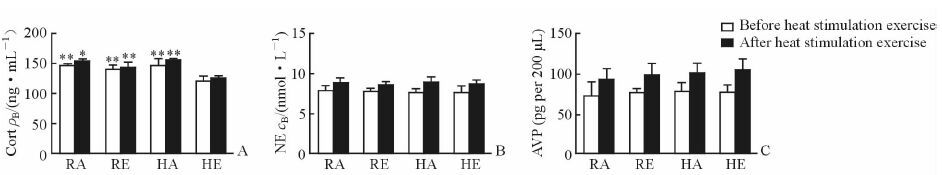
如图 2A示,HE组大鼠运动刺激前血浆Cort含量低于RA组、RE组和HA组 (P<0.01);接受高温高湿环境中高强度运动刺激30 min后,HE组大鼠血浆Cort含量仍低于RA组、RE组和HA组 (P<0.05,P<0.01)。各组间运动刺激前、运动刺激后血浆NE和AVP含量差异均无统计学意义 (图 2B和2C,P>0.05)。
|
图 2 湿热环境运动刺激前、后大鼠血浆中Cort、NE及AVP含量 Fig 2 Contents of plasma Cort, NE and AVP in rat before and after stimulation with exercise in hygrothermal environment A: Cort content; B: NE content; C: AVP content. RA: Room-temperature activity; RE: Room-temperature exercise; HA: High-temperature activity; HE: High-temperature exercise; Cort: Cortisol; NE: Norepinephrine; AVP: Arginine vasopressin. *P < 0.05, **P < 0.01 vs HE group. n=8, x±s |
2.3 湿热环境运动刺激对大鼠大脑皮质和心肌线粒体ATP合成酶、ROS及组织总抗氧化能力的影响
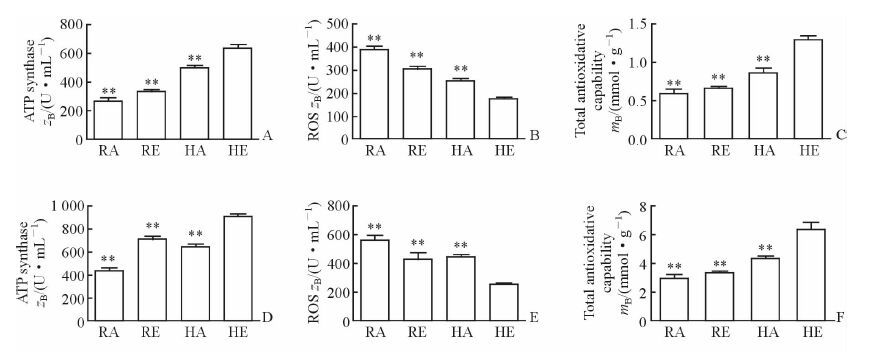
接受高温高湿环境中高强度运动刺激30 min后,HE组大鼠大脑皮质和心肌线粒体的ATP合成酶含量均高于RA组、RE组和HA组 (图 3A、3D,P<0.01);HE组大鼠大脑皮质和心肌线粒体中ROS的含量均低于RA组、RE组和HA组 (图 3B、3E,P<0.01);HE组大鼠大脑皮质和心肌组织的总抗氧化能力均高于RA组、RE组和HA组 (图 3C、3F,P<0.01)。
|
图 3 湿热环境运动刺激后大鼠大脑皮质和心肌线粒体ATP合成酶、ROS含量和组织总抗氧化能力 Fig 3 ATP synthase and ROS contents in mitochondrion and total antioxidative capacity in tissue from cerebral cortex and myocardium of rat after stimulation with exercise in hygrothermal environment A-C: ATP synthase, ROS contents and total antioxidative capacity of cerebral cortex, respectively; D-F: ATP synthase, ROS contents and total antioxidative capacity of myocardium, respectively. RA: Room-temperature activity; RE: Room-temperature exercise; HA: High-temperature activity; HE: High-temperature exercise; ATP: Adenosine triphosphate; ROS: Reactive oxygen species. **P < 0.01 vs HE group. n=6, x±s |
2.4 湿热环境运动刺激对大鼠大脑皮质和心肌线粒体超微结构的影响
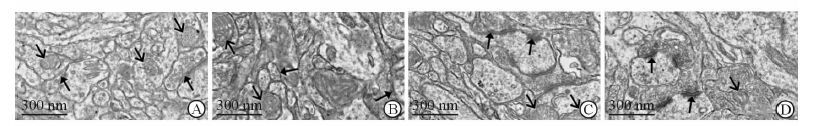
如图 4所示,接受高温高湿中高强度运动刺激30 min后,电镜下RA组大鼠大脑皮质线粒体膜严重溶解,线粒体嵴消失,突触前后膜完整性遭到破坏 (图 4A);RE组线粒体膜部分溶解,线粒体嵴折叠形成空泡状,突触结构模糊,突触囊泡数量稀少 (图 4B);HA组线粒体膜较为完整,嵴结构清晰,突触结构模糊,突触囊泡数量较少 (图 4C);HE组线粒体形态良好,线粒体嵴、突触结构清晰,前后膜结构完整,突触囊泡数量较多 (图 4D)。
|
图 4 湿热环境运动刺激后大鼠大脑皮质线粒体电镜图 Fig 4 Electron micrographs of mitochondrion from cerebral cortex of rat after stimulation with exercise in hygrothermal environment A: RA group; B: RE group; C: HA group; D: HE group. RA: Room-temperature activity; RE: Room-temperature exercise; HA: High-temperature activity; HE: High-temperature exercise. Empty-black arrows denote mitochondrion, full-black arrows denote cynapse |
如图 5所示,接受高温高湿中高强度运动刺激30 min后,RA组大鼠心肌电镜观察结果显示线粒体膜有部分溶解,线粒体嵴结构紊乱,Z线模糊,横小管变形 (图 5A);RE组线粒体膜结构较为完整,Z线部分溶解,横小管变形 (图 5B);HA组相邻线粒体间折叠形成空泡,线粒体嵴结构模糊,Z线明显变粗,横小管稍有变形 (图 5C);HE组线粒体形态结构良好,线粒体嵴结构和Z线清晰,横小管形态正常 (图 5D)。
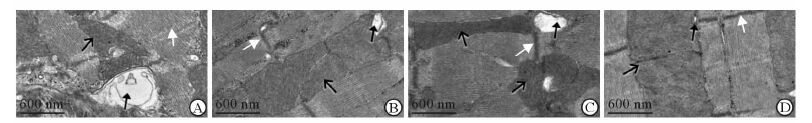
|
图 5 湿热环境下运动刺激后大鼠心肌线粒体电镜图 Fig 5 Electron micrographs of mitochondrion from myocardium of rat after stimulation with exercise in hygrothermal environment A: RA group; B: RE group; C: HA group; D: HE group. RA: Room-temperature activity; RE: Room-temperature exercise; HA: High-temperature activity; HE: High-temperature exercise. Empty-black arrows denote mitochondrion, full-black arrows denote transverse tubule, and full-white arrows denote Z line |
3 讨论
大量人体研究和我们的动物研究均发现,与单纯在高温环境下自由活动相比,在高温环境下从事一定强度的体力活动可明显缩短热习服的训练时间,即“热中练、动中练”的热习服效果明显优于单纯在高温下不从事体力活动的习服效果[13]。例如,单纯在热环境中自由活动的大鼠需要28 d才能获得习服,而我们的研究发现在热环境下进行中强度 (14 m/min) 的跑步锻炼,训练14 d即可使大鼠在热环境下 (温度36 ℃,相对湿度85%) 高强度运动 (28 m/min) 30 min后的死亡率明显降低[9]。本研究进一步证明,在高温环境下中强度运动训练14 d后再次接受湿热环境 (温度36 ℃,相对湿度85%) 中高强度运动 (21 m/min) 30 min后刺激,HE组大鼠在湿热环境运动刺激前、刺激后肛温升高的幅度明显低于RA组、RE组以及HA组,表明高温中强度运动的热习服训练降低了核心体温的升高幅度,减少了中暑的风险。HE组大鼠的体质量丢失率高于RA组,表明高温中强度运动的热习服训练使得HE组大鼠的出汗率大于RA组,增加了机体散热,减少了热应激损伤。Cort是反映机体压力和焦虑等水平的一种应激激素[14]。当机体受到热刺激、发热、情绪压力、抑郁等干扰时,血浆中Cort水平升高[14~15],表明机体的应激水平升高。本研究HE组大鼠血浆中Cort含量在湿热环境运动刺激前、刺激后均明显降低,说明高温中强度运动的热习服训练提高了大鼠对湿热运动刺激的耐受能力,降低了大鼠的应激水平,对机体起到了保护作用。
线粒体是细胞产生能量的重要细胞器,其形态和功能的良好状态是维持正常能量代谢过程的基本保证[16]。已有研究报道,热应激可破坏线粒体的形态结构并降低其氧化磷酸化的过程,使机体代谢过程中产生的有机酸不能迅速氧化分解为CO2和水并产生供机体利用的能量,同时有机酸的堆积和浓度的升高造成膜上关键酶的酶蛋白活性中心的拓扑结构发生变化,从而致使酶蛋白和底物的结合能力或催化能力降低,甚至导致线粒体膜结构和线粒体嵴遭到破坏[17~18];而Tamura等[10]报道,运动后进行全身热暴露训练获得热习服后,热习服组小鼠的体质量丢失率高于对照组,线粒体呼吸链的蛋白质含量增加,从而提高小鼠骨骼肌线粒体适应性。本研究的电镜观察结果显示,高温中强度热习服训练后的大鼠,在湿热环境中高强度运动刺激后,其大脑皮质和心肌线粒体膜结构和嵴的完整性得到了最大程度的改善。线粒体膜结构的完整性为附着于其上的ATP合成酶的功能正常发挥起到了重要的保护作用,从而提高了大脑皮质和心肌线粒体ATP合成酶的含量。ATP合成酶含量的增加可能预示着线粒体催化合成ATP的能力增强,使更多的ADP转化为ATP以提供更多的能量[19~20]。然而,细胞内高浓度的ROS蓄积则导致DNA损伤和细胞死亡[21]。线粒体产能能力的增强和组织总抗氧化能力的提高,可有效降低神经元和心肌细胞对氧化应激损伤的耐受能力,减轻了对细胞结构的氧化损伤,从而使ROS水平下降,降低机体的应激水平。因此,高温中强度运动的热习服训练阻断了湿热环境运动刺激导致的“线粒体膜结构损伤→产能能力下降→氧化应激损伤加剧→线粒体膜结构进一步损伤”的恶性循环。
综上所述,高温中强度运动的热习服训练保护了大脑皮质神经元和心肌细胞线粒体结构,增强了线粒体的氧化呼吸功能,降低了细胞的氧化应激水平,使得应激激素Cort含量下降,从而降低大脑皮质神经元和心肌细胞对湿热环境中高强度运动的应激水平,提高其耐受能力。本研究为湿热环境作业提供了实验依据。
| [1] | PRYOR R R, BENNETT B L, O'CONNOR F G, YOUNG J M, ASPLUND C A. Medical evaluation for exposure extremes:heat[J]. Wilderness Environ Med, 2015, 26(4 Suppl): S69–S75. |
| [2] | YAMAZAKI F. Effectiveness of exercise-heat acclimation for preventing heat illness in the workplace[J]. J UOEH, 2013, 35: 183–192. DOI: 10.7888/juoeh.35.183 |
| [3] | MCDONALD F B, CHANDRASEKHARAN K, WILSON R J A, HASAN S U. Cardiorespiratory control and cytokine profile in response to heat stress, hypoxia, and lipopolysaccharide (LPS) exposure during early neonatal period[J/OL]. Physiol Rep, 2016, 4:e12688. doi:10.14814/phy2.12688. |
| [4] | HOSTLER D, COLBURN D, RITTENBERGER J C, REIS S E. Effect of two work-to-rest ratios on cardiovascular, thermal, and perceptual responses during fire suppression and recovery[J]. Prehosp Emerg Care, 2016, 20: 681–687. DOI: 10.3109/10903127.2016.1168890 |
| [5] | CUI J, ARBAB-ZADEH A, PRASAD A, DURAND S, LEVINE B D, CRANDALL C G. Effects of heat stress on thermoregulatory responses in congestive heart failure patients[J]. Circulation, 2005, 112: 2286–2292. DOI: 10.1161/CIRCULATIONAHA.105.540773 |
| [6] | BAIN A R, NYBO L, AINSLIE P N. Cerebral vascular control and metabolism in heat stress[J]. Compr Physiol, 2015, 5: 1345–1380. |
| [7] | SLIMEN I B, NAJAR T, GHRAM A, DABBEBI H, BEN MRAD M, ABDRABBAH M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review[J]. Int J Hyperthermia, 2014, 30: 513–523. DOI: 10.3109/02656736.2014.971446 |
| [8] | SCHWIMMER H, GERSTBERGER R, HOROWITZ M. Heat acclimation affects the neuromodulatory role of AngⅡ and nitric oxide during combined heat and hypohydration stress[J]. Brain Res Mol Brain Res, 2004, 130: 95–108. DOI: 10.1016/j.molbrainres.2004.07.011 |
| [9] | 李玉祥, 陈新民, 梁涵, 安合定, 马文领, 史仍飞. 不同热习服条件对大鼠湿热环境下运动能力的影响[J]. 海军医学杂志, 2016, 37: 4–8. DOI: 10.3969/j.issn.1009-0754.2016.01.002 |
| [10] | TAMURA Y, MATSUNAGA Y, MASUDA H, TAKAHASHI Y, TERADA S, HOSHINO D, et al. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle[J]. Am J Physiol Regul Integr Comp Physiol, 2014, 307: R931–R943. DOI: 10.1152/ajpregu.00525.2013 |
| [11] | 赵荣华, 刘进娜, 李聪, 张敬升, 王帮众, 么元超, 等. 肝郁、脾虚和肝郁脾虚证模型大鼠下丘脑-垂体-肾上腺轴变化及柴疏四君汤的干预效应[J]. 中国中西医结合杂志, 2015, 35: 834–838. |
| [12] | LESNIK C, ARAVA Y. Isolation of mRNAs associated with yeast mitochondria to study mechanisms of localized translation[J/OL]. J Vis Exp, 2014:e51265. doi:10.3791/51265. |
| [13] | 邱仞之. 环境高温与热损伤[M]. 北京: 军事医学科学出版社, 2000. |
| [14] | SOETRISNO, SULISTYOWATI S, ARDHIANTO A, HADI S. The effect of logotherapy on the expressions of cortisol, HSP70, beck depression inventory (BDI), and pain scales in advanced cervical cancer patients[J]. Health Care Women Int, 2016, 20: 1–9. |
| [15] | LYNN B M, MINSON C T, HALLIWILL J R. Fluid replacement and heat stress during exercise alter post-exercise cardiac haemodynamics in endurance exercise-trained men[J]. J Physiol, 2009, 587(Pt 14): 3605–3617. |
| [16] | VOOS W, JAWOREK W, WILKENING A, BRUDEREK M. Protein quality control at the mitochondrion[J]. Essays Biochem, 2016, 60: 213–225. DOI: 10.1042/EBC20160009 |
| [17] | VOLTOLINI D M, DEL VESCO A P, GASPARINO E, GUIMARÃES S E, OLIVEIRA NETO A R, BATISTA E, et al. Mitochondrial gene expression in the liver and muscle of high and low feed efficiency Japanese quail layers subjected to different environmental temperatures[J]. Genet Mol Res, 2014, 13: 4940–4948. DOI: 10.4238/2014.July.4.8 |
| [18] | TRINEI M, MIGLIACCIO E, BERNARDI P, PAOLUCCI F, PELICCI P, GIORGIO M. p66Shc, mitochondria, and the generation of reactive oxygen species[J]. Methods Enzymol, 2013, 528: 99–110. DOI: 10.1016/B978-0-12-405881-1.00006-9 |
| [19] | TEIXEIRA F K, SANCHEZ C G, HURD T R, SEIFERT J R, CZECH B, PREALL J B, et al. ATP synthase promotes germ cell differentiation independent of oxidative phosphorylation[J]. Nat Cell Biol, 2015, 17: 689–696. DOI: 10.1038/ncb3165 |
| [20] | HAHN A, PAREY K, BUBLITZ M, MILLS D J, ZICKERMANN V, VONCK J, et al. Structure of a complete ATP synthase dimer reveals the molecular basis of inner mitochondrial membrane morphology[J]. Mol Cell, 2016, 63: 445–456. DOI: 10.1016/j.molcel.2016.05.037 |
| [21] | BLASER H, DOSTERT C, MAK T W, BRENNER D. TNF and ROS crosstalk in inflammation[J]. Trends Cell Biol, 2016, 26: 249–261. DOI: 10.1016/j.tcb.2015.12.002 |
 2017, Vol. 38
2017, Vol. 38


