血管平滑肌细胞 (vascular smooth muscle cell,VSMC) 是血管壁的重要组成部分,其通过收缩和舒张调节正常发育成熟个体的血管内径及血压。VSMC不是一种终末分化的细胞,其可受周围环境因素影响而发生表型转化[1]。正常成熟的VSMC以收缩型为主,该型细胞中VSMC表型标记物——α-平滑肌肌动蛋白 (α-smooth muscle actin,α-SMA) 和肌球蛋白重链11(myosin heavy chain 11,MYH11) 表达丰富,且细胞增殖和迁移能力弱。在血管内膜损伤、炎症因子刺激等病理条件下,VSMC会发生“去分化”,由收缩型向合成型转化。合成型VSMC中α-SMA和MYH11的表达减少,但细胞增殖和迁移能力强[2]。目前认为,VSMC表型转化在动脉粥样硬化、高血压、血管成形或旁路移植术后再狭窄等多种心血管疾病中发挥重要作用[3-4]。
Ets差异基因5(E-twenty-six variant gene 5,ETV5) 是ETS家族中PEA3亚家族的一种转录因子。ETV5调控多种基因的表达,并在细胞的增殖、分化、迁移、凋亡和上皮-间质转化 (epithelial-to-mesenchymal transition,EMT) 等生物学过程中发挥作用[5-6]。Monge等[7]研究发现ETV5能够激活基质金属蛋白酶2(matrix metalloproteinase-2,MMP-2) 表达,促进子宫内膜癌的早期转移。Colas等[5]研究报道ETV5能够诱导子宫内膜癌细胞EMT,从而增强细胞的迁移和侵袭能力。
作为表型转化的一种常见方式,EMT与VSMC的表型转化在分子调控机制、细胞生物学行为的改变等方面具有很大的相似性[8],且ETV5下游因子MMP-2是调控VSMC增殖和迁移的关键因子之一[1]。但关于ETV5在VSMC表型转化中的作用研究目前未见报道。本研究采用过表达和敲低VSMC中ETV5的方法,观察ETV5在VSMC表型转化过程中的作用。
1 材料和方法 1.1 细胞培养和处理采用含有平滑肌细胞生长因子的Medium 231培养液 (Invitrogen公司) 培养人VSMC (Cascade Biologics公司,C-007-5C)。待细胞生长融合后,用0.25%胰蛋白酶在37 ℃下消化传代,取第3~6代VSMC用于实验。
当细胞生长融合至60%时,分别采用ETV5过表达腺病毒载体 (Ad-ETV5组) 或绿色荧光蛋白 (GFP) 过表达腺病毒载体 (Ad-GFP组) 转染VSMC 48 h,后用于后续实验。
采用Lipofectamine 2000转染试剂盒 (Invitrogen公司) 分别用人ETV5特异性小干扰RNA (siRNAETV5) 及其随机阴性序列 (siRNAscramble) 按照100 nmol/L的终浓度转染VSMC 6 h,更换培养液继续培养24 h后,加入血小板源性生长因子 (PDGF)-BB (20 ng/mL) 处理24 h诱导VSMC表型转化。
1.2 蛋白质印迹法检测ETV5、α-SMA和MYH11蛋白表达PBS洗涤细胞3次后,采用含有1 mmol/L蛋白酶抑制剂的SDS细胞裂解液 (上海碧云天生物技术有限公司) 裂解细胞。裂解充分后,4 ℃ 12 000×g离心5 min,收集上清。采用BCA法检测蛋白浓度,根据测得的蛋白浓度调整样品的上样体积,使每个SDS聚丙烯酰胺凝胶孔道中的蛋白总含量为50 μg。凝胶中的蛋白依次经过电泳分离、转膜 (PVDF膜)、5%脱脂奶粉溶液封闭1 h后,4 ℃过夜分别标记抗ETV5一抗 (Proteintech公司,13011-1-AP)、抗α-SMA一抗 (Boster公司,BM0002)、抗MYH11一抗 (Santa Cruz公司,sc-6956) 和抗β-actin一抗 (Proteintech公司,60008-1-Ig)。次日室温标记二抗1 h。采用ECL化学发光仪显像拍照,采用Image J软件计算灰度值。
1.3 qPCR检测ETV5、α-SMA和MYH11 mRNA表达采用TRIzol法抽提各组VSMC的总RNA。按照TaKaRa公司反转录试剂盒操作说明进行反转录反应,获得cDNA。按照TaKaRa公司染料法荧光定量试剂盒进行qPCR反应。GAPDH为内参照基因。实验所用的引物序列:ETV5上游5′-CAG TCA ACT TCA AGA GGC TTG G-3′,下游5′-TGC TCA TGG CTA CAA GAC GAC-3′;α-SMA上游5′-GTG TTG CCC CTG AAG AGC AT-3′,下游5′-GCT GGG ACA TTG AAA GTC TCA-3′;MYH11上游5′-CGC CAA GAG ACT CGT CTG G-3′,下游5′-TCT TTC CCA ACC GTG ACC TTC-3′;GAPDH上游5′-CTG GGC TAC ACT GAG CAC C-3′,下游5′-AAG TGG TCG TTG AGG GCA AT-3′。
1.4 平板划痕实验检测VSMC的迁移能力将灭菌直尺置于6孔板上方,用200 μL移液器枪头紧贴直尺,在板底中央划痕,PBS洗板底3次去除划掉的细胞残渣,于镜下随机选取3个视野拍照,孵育24 h再次拍照。计数24 h时两条直线之间的细胞个数,将其作为迁移能力的定量指标。
1.5 CCK-8法检测VSMC的增殖能力将VSMC消化、重悬、计数后,以2×104/mL的细胞密度接种到96孔板中,每孔100 μL,每组设3个复孔。待细胞贴壁后,每孔加入10 μL CCK-8溶液,孵育2 h。酶标仪检测450 nm波长处的光密度 (D) 值。实验重复3次。
1.6 统计学处理采用SPSS 19.0软件进行数据分析。计量资料以x±s表示,采用t检验或方差分析 (ANOVA) 进行组间比较。检验水准 (α) 为0.05。
2 结果 2.1 过表达ETV5下调VSMC中α-SMA和MYH11的表达如图 1A所示,光镜下可见体外培养的VSMC融合度约80%,相同视野下观察到Ad-ETV5转染细胞后,超过70% VSMC发出绿色荧光;此外,蛋白质印迹结果显示VSMC转染Ad-ETV5后ETV5蛋白的表达增加 (0.64±0.02 vs 0.13±0.01, P < 0.05;图 1B),提示ETV5在VSMC中成功过表达。qPCR和蛋白质印迹结果均显示ETV5过表达能够抑制VSMC中α-SMA和MYH11的表达 (P < 0.05,图 1B、1C)。

|
图 1 ETV5过表达抑制VSMC中α-SMA和MYH11的表达 Fig 1 Overexpression of ETV5 inhibits expressions of α-SMA and MYH11 in VSMCs A: VSMCs transfected with Ad-ETV5 emitting green fluorescence; B: The results of Western blotting analysis showed that the protein expressions of α-SMA and MYH11 in Ad-ETV5 group were lower than those in Ad-GFP group; C: The results of qPCR showed that the mRNA levels of α-SMA and MYH11 in Ad-ETV5 group were lower than those in Ad-GFP group. ETV5: E-twenty-six variant gene 5; GFP: Green fluorescent protein; VSMC: Vascular smooth muscle cell; α-SMA: α-Smooth muscle actin; MYH11: Myosin heavy chain 11. *P < 0.05 vs Ad-GFP group. n=3, x±s. Original magnification: ×100 (A) |
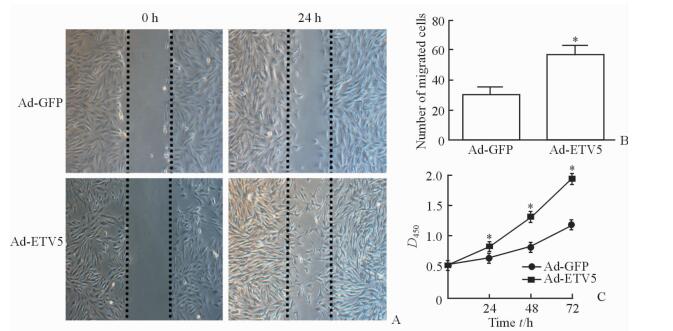
2.2 过表达ETV5促进VSMC的迁移和增殖
平板划痕实验结果显示,与Ad-GFP组相比,过表达ETV5能促进VSMC的迁移 (迁移细胞数:57.0±5.2 vs 29.7±5.2,P < 0.05;图 2A、2B)。CCK-8法检测结果显示,Ad-ETV5组VSMC在24、48和72 h 3个时间点的D值均高于Ad-GFP组 (P < 0.05,图 2C),提示ETV5能够增强VSMC的增殖能力。
|
图 2 ETV5过表达促进VSMC的迁移和增殖 Fig 2 Overexpression of ETV5 promotes proliferation and migration of VSMCs A, B: Compared with Ad-GFP group, overexpression of ETV5 promotes the migration of VSMCs by scratch-wound assay; C: Compared with Ad-GFP group, overexpression of ETV5 promotes the proliferation of VSMCs by CCK-8 assay. ETV5: E-twenty-six variant gene 5; GFP: Green fluorescent protein; VSMC: Vascular smooth muscle cell. *P < 0.05 vs Ad-GFP group. n=3, x±s. Original magnification: ×100 (A) |
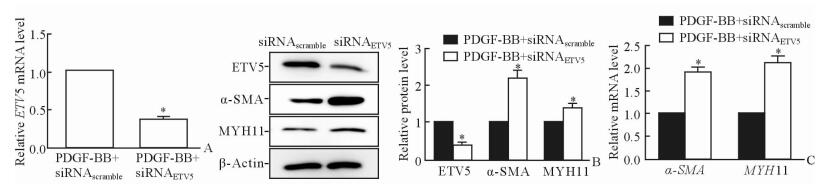
2.3 敲低ETV5抑制PDGF-BB介导的α-SMA和MYH11下调
与PDGF-BB+siRNAscramble组相比,PDGF-BB+siRNAETV5组VSMC中ETV5 mRNA (图 3A) 和蛋白 (图 3B) 表达降低 (P < 0.05)。敲低VSMC中ETV5的表达后α-SMA和MYH11的mRNA和蛋白表达较PDGF-BB+siRNAscramble组均增加 (P < 0.05;图 3B、3C),提示敲低ETV5能够抑制PDGF-BB对VSMC表型标记物α-SMA和MYH11的下调作用。
|
图 3 ETV5敲低抑制PDGF-BB引起的VSMC中α-SMA和MYH11表达下调 Fig 3 Knockdown of ETV5 inhibits the down-regulation of α-SMA and MYH11 of VSMCs induced by PDGF-BB A: Compared with PDGF-BB+siRNAscramble group, mRNA level of ETV5 of VSMCs was reduced in PDGF-BB+siRNAETV5 group by qPCR; B: Knockdown of ETV5 upregulated protein expressions of α-SMA and MYH11 by Western blotting analysis; C: Knockdown of ETV5 upregulated mRNA expressions of α-SMA and MYH11 by qPCR. ETV5: E-twenty-six variant gene 5; GFP: Green fluorescent protein; PDGF: Platelet-derived growth factor; VSMC: Vascular smooth muscle cell; α-SMA: α-Smooth muscle actin; MYH11: Myosin heavy chain 11. *P < 0.05 vs PDGF-BB+siRNAscramble group. n=3, x±s |
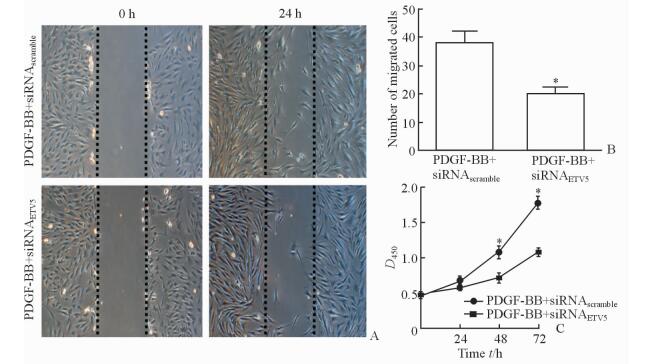
2.4 敲低ETV5抑制PDGF-BB对VSMC增殖和迁移的促进作用
平板划痕实验结果显示,与PDGF-BB+siRNAscramble组相比,敲低ETV5能够抑制PDGF-BB对VSMC迁移能力的促进作用 (迁移细胞数:38.0±4.4 vs 20.0±2.6,P < 0.05;图 4A、4B)。此外,CCK-8法检测结果显示,敲低ETV5还能抑制PDGF-BB对VSMC的促增殖作用 (P < 0.05,图 4C)。
|
图 4 ETV5敲低抑制PGDF-BB的促VSMC迁移和增殖作用 Fig 4 Knockdown of ETV5 inhibits proliferation and migration of VSMCs induced by PDGF-BB A, B: Knockdown of ETV5 inhibited the migration of VSMCs by scratch-wound assay; C: Knockdown of ETV5 inhibited the proliferation of VSMCs by CCK-8 assay. ETV5: E-twenty-six variant gene 5; GFP: Green fluorescent protein; PDGF: Platelet-derived growth factor; VSMC: Vascular smooth muscle cell. *P < 0.05 vs PDGF-BB+siRNAscramble group. n=3, x±s. Original magnification: ×100 (A) |
3 讨论
与骨骼肌和心肌等终末分化的肌细胞不同,VSMC具有很强的表型可塑性,其表型可受到动脉粥样硬化、高血压、血管损伤等多种病理因素的影响而发生转化[9]。近年来,对VSMC的表型转化有了新的研究进展。Shankman等[10]研究发现,VSMC表型转化在动脉粥样硬化损伤进展、斑块形成以及调节斑块稳定性等方面具有关键作用,抑制VSMC的表型转化能够提高动脉粥样硬化斑块的稳定性。O’Brien等[11]研究发现,VSMC增殖和迁移能力的增强在血管内膜增生形成过程中发挥着重要作用,而内膜增生是动脉粥样硬化、支架内狭窄和冠脉动脉旁路移植术后静脉桥狭窄等疾病的关键病理学过程。因此,研究VSMC表型转化的调控机制对于明确多种心血管疾病的分子生物学机制,进而对提供新的潜在治疗靶点具有重要意义。
ETS家族是一个由高度保守基因组成的群体,在不同组织细胞的增殖、分化、迁移以及侵袭过程中发挥着极其重要的作用。Kumar-Sinha等[12]研究表明,过表达ETV5可使前列腺癌细胞更具有侵袭性。Colas等[5]发现ETV5可以促进子宫内膜癌EMT,且通过调节ZEB1和E-钙黏蛋白促进子宫内膜癌细胞-细胞和细胞-基底接触的重组。此外,ETV5在EMT和促进子宫内膜肿瘤细胞的迁移、侵袭能力增强过程中发挥重要作用[13]。
既往研究证实PDGF-BB能够抑制VSMC表型标记物的表达,并且能够增强VSMC的增殖和迁移能力[1, 4]。本研究采用PDGF-BB刺激VSMC构建VSMC表型转化细胞模型。经研究发现,ETV5过表达能导致人VSMC分化表型标记物下调,并促进VSMC的迁移和增殖。通过siRNA敲低ETV5在VSMC中的表达,结果发现ETV5的敲低能够抑制PDGF-BB诱导的分化表型标记物的表达下调,还能抑制PDGF-BB诱导的VSMC的增殖和迁移。本研究提示ETV5是有效抑制VSMC表型转化的潜在靶点,为动脉粥样硬化及血管支架内狭窄等疾病提供了新的治疗靶点。
Akagi等[14]研究发现,ETV5是转录因子OCT4的重要下游因子之一。Cherepanova等[15]证实了OCT4介导的VSMC由中膜向内膜迁移在动脉粥样硬化斑块形成过程中发挥了重要作用。Mak等[16]的研究结果提示,microRNA-145能够抑制ETV5的表达;而microRNA-145是调控VSMC表型转化的关键因子,其在VSMC中的表达会因动脉粥样硬化、血管损伤等多种血管疾病而出现显著下调,进而引起VSMC表型标记物的降低和细胞增殖能力的提高[17]。以上研究提示,在病理条件下ETV5可能会受到多种分子机制的调控,上调其在VSMC中的表达,进而导致VSMC向合成型转变,但具体调控机制仍有待进一步研究。
吴素慧等[18]在宫颈癌组织中发现ETV5与MMP-7的表达呈正相关。此外,Monge等[7]在肿瘤研究中发现,转录因子ETV5可直接结合MMP-2的启动子区而激活MMP-2的转录表达。过表达ETV5能够上调MMP-2在宫颈癌和软骨肉瘤细胞中的表达,从而增强癌细胞的侵袭转移和增殖能力[7, 19]。鉴于MMP-2已被证实是多种血管疾病中促进平滑肌细胞增殖和迁移的关键因子,我们推测ETV5可能部分通过上调VSMC中MMP-2的表达发挥其促增殖和迁移的生物学作用,未来需进一步验证。
综上所述,ETV5在病理条件下,可能会受到多种分子机制的调控,上调其在VSMC中的表达,进而导致VSMC向合成型表型转变,并调节VSMC的增殖和迁移。然而,ETV5介导VSMC表型转化的更深入的分子生物学机制仍有待今后的进一步研究。
| [1] | OWENS G K, KUMAR M S, WAMHOFF B R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease[J]. Physiol Rev, 2004, 84: 767–801. DOI: 10.1152/physrev.00041.2003 |
| [2] | ALEXANDER M R, OWENS G K. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease[J]. Annu Rev Physiol, 2012, 74: 13–40. DOI: 10.1146/annurev-physiol-012110-142315 |
| [3] | RZUCIDLO E M, MARTIN K A, POWELL R J. Regulation of vascular smooth muscle cell differentiation[J]. J Vasc Surg, 2007, 45(Suppl A): A25–A32. |
| [4] | LIU R, JIN Y, TANG W H, QIN L, ZHANG X, TELLIDES G, et al. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity[J]. Circulation, 2013, 128: 2047–2057. DOI: 10.1161/CIRCULATIONAHA.113.002887 |
| [5] | COLAS E, MUINELO-ROMAY L, ALONSO-ALCONADA L, LLAURADO M, MONGE M, BARBAZAN J, et al. ETV5 cooperates with LPP as a sensor of extracellular signals and promotes EMT in endometrial carcinomas[J]. Oncogene, 2012, 31: 4778–4788. DOI: 10.1038/onc.2011.632 |
| [6] | SHARROCKS A D. The ETS-domain transcription factor family[J]. Nat Rev Mol Cell Biol, 2001, 2: 827–837. DOI: 10.1038/35099076 |
| [7] | MONGE M, COLAS E, DOLL A, GONZALE M, GIL-MORENO A, PLANAGUMA J, et al. ERM/ETV5 up-regulation plays a role during myometrial infiltration through matrix metalloproteinase-2 activation in endometrial cancer[J]. Cancer Res, 2007, 67: 6753–6759. DOI: 10.1158/0008-5472.CAN-06-4487 |
| [8] | SAITO A. EMT and EndMT: regulated in similar ways?[J]. J Biochem, 2013, 153: 493–495. DOI: 10.1093/jb/mvt032 |
| [9] | YOSHIDA T, OWENS G K. Molecular determinants of vascular smooth muscle cell diversity[J]. Circ Res, 2005, 96: 280–291. DOI: 10.1161/01.RES.0000155951.62152.2e |
| [10] | SHANKMAN L S, GOMEZ D, CHEREPANOVA O A, SALMON M, ALENCAR G F, HASKINS R M, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis[J]. Nat Med, 2015, 21: 628–637. DOI: 10.1038/nm.3866 |
| [11] | O'BRIEN E R, MA X, SIMARD T, POURDJABBAR A, HIBBERT B. Pathogenesis of neointima formation following vascular injury[J]. Cardiovasc Hematol Disord Drug Targets, 2011, 11: 30–39. DOI: 10.2174/187152911795945169 |
| [12] | KUMAR-SINHA C, TOMLINS S A, CHINNAIYAN A M. Recurrent gene fusions in prostate cancer[J]. Nat Rev Cancer, 2008, 8: 497–511. DOI: 10.1038/nrc2402 |
| [13] | ALONSO-ALCONADA L, ERITJA N, MUINELO-ROMAY L, BARBAZAN J, LOPEZ-LOPEZ R, MATIAS-GUIU X, et al. ETV5 transcription program links BDNF and promotion of EMT at invasive front of endometrial carcinomas[J]. Carcinogenesis, 2014, 35: 2679–2686. DOI: 10.1093/carcin/bgu198 |
| [14] | AKAGI T, KUURE S, URANISHI K, KOIDE H, COSTANTINI F, YOKOTA T. ETS-related transcription factors ETV4 and ETV5 are involved in proliferation and induction of differentiation-associated genes in embryonic stem (ES) cells[J]. J Biol Chem, 2015, 290: 22460–22473. DOI: 10.1074/jbc.M115.675595 |
| [15] | CHEREPANOVA O A, GOMEZ D, SHANKMAN L S, SWIATLOWSKA P, WILLIAMS J, SARMENTO O F, et al. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective[J]. Nat Med, 2016, 22: 657–665. DOI: 10.1038/nm.4109 |
| [16] | MAK I W, SINGH S, TURCOTTE R, GHERT M. The epigenetic regulation of SOX9 by miR-145 in human chondrosarcoma[J]. J Cell Biochem, 2015, 116: 37–44. DOI: 10.1002/jcb.v116.1 |
| [17] | CHENG Y, LIU X, YANG J, LIN Y, XU D Z, LU Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation[J]. Circ Res, 2009, 105: 158–166. DOI: 10.1161/CIRCRESAHA.109.197517 |
| [18] | 吴素慧, 张静, 李颖, 李建民. ETV5与MMP-7在早期宫颈癌组织中的表达及其在转移中的作用[J]. 癌症, 2006, 25: 315–319. DOI: 10.3321/j.issn:1000-467X.2006.03.011 |
| [19] | POWER P F, MAK I W, SINGH S, POPOVIC S, GLADDY R, GHERT M. ETV5 as a regulator of matrix metalloproteinase 2 in human chondrosarcoma[J]. J Orthop Res, 2013, 31: 493–501. DOI: 10.1002/jor.v31.3 |
 2017, Vol. 38
2017, Vol. 38


