构建具有生物活性并能成功修复和重塑骨缺损的骨修复材料一直是研究者不懈努力的方向和目标,而骨形态发生蛋白2 (bone morphogenetic protein 2, BMP-2) 在骨修复中扮演了重要角色[1-3]。BMP-2诱导成骨分化效果明显,且已被用于临床,但BMP-2在诱导成骨分化时常表现出剂量依赖性,且易失活、易降解[4-5]。所以,如何使高生物活性的BMP-2在骨组织工程中长效使用越来越得到重视。
许多生物相容性良好的高分子材料制备的海绵状多孔支架已广泛应用于骨组织工程的研究中,如聚己内酯 (polycaprolactone,PCL)[6]。材料支架加工常因蛋白质结构破坏导致BMP-2失活,且大部分高分子材料都是疏水性材料,与水溶性的BMP-2难以兼容。因此,如何将大量的BMP-2负载在高分子材料支架上且长时间发挥作用仍是一个难题。
文献报道利用磷脂将胰岛素加载于纳米材料中可制备出一种能够大量负载胰岛素、且长期维持胰岛素活性的胰岛素制剂[7]。以此为启发,本研究尝试利用大豆磷脂 (phospholipid,PL) 修饰BMP-2制备一种可大量负载BMP-2、同时能长时间缓释有活性BMP-2的三维多孔PCL-B/P复合支架,并通过检测该支架上的人源骨髓间充质干细胞 (bone marrow stromal cell,BMSC) 的增殖和成骨分化能力探讨其在骨组织工程中的临床应用。
1 材料和方法 1.1 支架的制备及观察将10 mg的BMP-2(美国赛默飞公司) 与5 mg的大豆PL[生工生物工程 (上海) 股份有限公司]同时分散在二氯甲烷中,依次经过匀浆处理30 s、通风干燥12 h、冷冻干燥6 h得到PL修饰的BMP-2复合体,即BMP-2/PL复合体 (B/P)。
将1.5 g的PCL (相对分子质量为25 000,山东岱岗生物科技有限公司) 溶解在30 mL的1, 4-二氧六烷中形成均匀溶液,再加入15 mg的B/P混合得到PCL-B/P混合液。将混合液倒入30 mL的玻璃烧杯中迅速液氮冷冻,然后置于冷冻干燥机中低温干燥2 d,除去残余的有机溶剂,可得到PCL-B/P复合支架。同样方法制备负载未被PL修饰的BMP-2的PCL-B传统支架,作为对照组。
使用扫描电子显微镜 (scanning electron microscope,SEM;日本电子株式会社) 观察PCL-B/P复合支架和PCL-B传统支架的微观结构。
1.2 酶联免疫吸附试验 (ELISA) 检测支架的BMP-2的释放曲线将制备的支架切割成5 mm×5 mm×5 mm的小块,分别放置于50 mL PBS的离心管内,于37 ℃培养箱中连续释放22 d。两组分别定期 (PCL-B/P复合支架:0、0.25、0.5、0.75、1、1.5、2、3、4、5、6、7、8、9、10、12、14、16、18、20和22 d;PCL-B传统支架:0、0.25、0.5、0.75、1、1.5、2、3、4、5、6、7、14、20和22 d) 取0.2 mL,ELISA检测BMP-2的含量,根据公式计算支架BMP-2的释放率 (R):R (%)=BMPR/BMPT×100%,其中BMPR为复合支架释放的BMP-2含量,BMPT为复合支架样品负载的BMP-2含量。
1.3 CCK-8法检测支架上BMSC的增殖能力将复合支架及传统支架分别切割成48孔板的孔径大小,经紫外线照射2 h后放置在48孔板中,种植人源BMSC (购买于中国科学院上海干细胞库),细胞密度为2×105/孔,于细胞培养的第7、14和21天,采用CCK-8试剂盒 (美国赛默飞公司) 检测支架上BMSC的增殖能力。利用酶标仪测定450 nm波长处的光密度 (D) 值。每个样品取3个平行样。
1.4 碱性磷酸酶 (alkaline phosphatase,ALP) 活性及实时定量PCR (qPCR) 检测支架上BMSC成骨分化能力按照ALP试剂盒 (美国赛默飞公司) 操作说明检测细胞培养的第7、14和21天时PCL-B/P复合支架和PCL-B传统支架上BMSC的ALP活性。
待细胞培养的第7、14和21天时,消化、中和、离心获得支架上的细胞,采用qPCR检测BMSC的3种成骨分化基因:Ⅰ型胶原蛋白 (collagen typeⅠ,COL-Ⅰ)、骨钙素 (osteocalcin,OC) 和骨桥蛋白 (osteopontin,OPN)。使用RNA提取试剂盒得到总RNA,经反转录反应得到cDNA并定量,然后以cDNA为模板、GAPDH为内参照进行qPCR反应,反应体系为:6.25 μL 2×PCR Master Mix、0.5 μL Primer-F、0.5 μL Primer-R、2.5 μL cDNA,再加入ddH2O至总体积为12.5 μL。引物序列如下:COL-Ⅰ上游5′-GAC GAA GAC ATC CCA CCA AT-3′,下游5′-AGA TCA CGT CAT CGC ACA AC-3′;OC上游5′-GTG CAG CCT TTG TGT CCA A-3′,下游5′-GCT CAC ACA CCT CCC TCC T-3′;OPN上游5′-ACT GAT TTT CCC ACG GAC CT-3′,下游5′-TCA GGG TAC TGG ATG TCA GG-3′;GAPDH上游5′-CAC ATC TCC AGG AGC GAG-3′,下游5′-TCA CGC CAC AGT TTC CCG GA-3′。每个样品取3个平行样。
1.5 统计学处理数据均采用SPSS 22.0软件进行分析,计量资料用x±s表示,两组间比较采用t检验。检验水准 (α) 为0.05。
2 结果 2.1 复合支架微观结构负载了PL修饰BMP-2的PCL-B/P复合支架的显微结构 (图 1A) 呈多孔海绵状,与负载了PL未修饰BMP-2的PCL-B传统支架 (图 1B) 的结构类似,平均孔径分别为 (241.4±31.2) μm和 (236.9±45.5) μm。

|
图 1 PCL-B/P复合支架 (A) 和PCL-B传统支架 (B) 的SEM图 Fig 1 Scanning electron microscope (SEM) images of polycaprolactone (PCL)-B/P composite scaffold (A) and PCL-B traditional scaffold (B) |
2.2 两种支架的BMP-2缓释效果比较
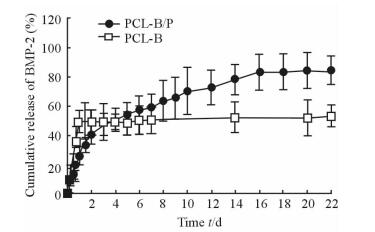
如图 2所示,在缓释检测的最初2 d内PCL-B/P复合支架存在一个很弱的突释,累积释放率约为35%;第2天后PCL-B/P复合支架持续释放BMP-2,至第14天时BMP-2的累积释放率约为75%,并最终稳定在85%左右。而PCL-B传统支架在1 d内突释BMP-2高达50%,并在之后的21 d都基本维持在50%,没有缓释现象。
|
图 2 PCL-B/P复合支架和PCL-B传统支架的BMP-2缓释能力 Fig 2 Slow-release behaviors of BMP-2 in PCL-B/P composite scaffold and PCL-B traditional scaffold BMP-2: Bone morphogenetic protein 2; PCL: Polycaprolactone. n=3, x±s |
2.3 两种支架上BMSC的增殖能力比较
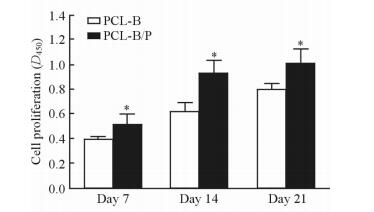
在细胞培养的第7、14和21天,PCL-B/P复合支架上的细胞增殖能力均优于PCL-B传统支架,差异均有统计学意义 (P < 0.05,图 3)。
|
图 3 PCL-B/P复合支架和PCL-B传统支架上BMSC的增殖能力 Fig 3 Proliferation ability of BMSC on PCL-B/P composite scaffold and PCL-B traditional scaffold PCL: Polycaprolactone; BMSC: Bone marrow stromal cell. *P < 0.05 vs PCL-B traditional scaffold. n=3, x±s |
2.4 两种支架上BMSC的成骨分化能力比较
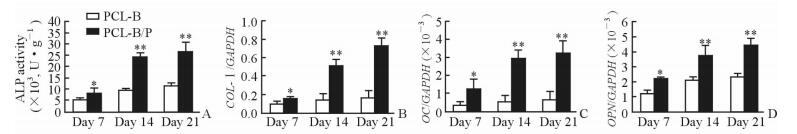
如图 4所示,培养第7、14和21天时PCL-B/P复合支架上BMSC的ALP活性均高于PCL-B传统支架,差异均有统计学意义 (P < 0.05),且COL-Ⅰ、OC和OPN mRNA的表达水平也均高于PCL-B传统支架,差异均有统计学意义 (P < 0.05),说明缓释BMP-2的PCL-B/P复合支架较PCL-B传统支架更有利于BMSC的成骨诱导分化。
|
图 4 PCL-B/P复合支架和PCL-B传统支架上BMSC的成骨分化能力 Fig 4 Osteogenic differentiation ability of BMSC on PCL-B/P composite scaffold and PCL-B traditional scaffold A: ALP activity by ALP Kit; B-D: The mRNA expressions of COL-Ⅰ(B), OC (C) and OPN (D) by qPCR. PCL: Polycaprolactone; BMSC: Bone marrow stromal cell; ALP: Alkaline phosphatase; COL-Ⅰ: Collagen typeⅠ; OC: Osteocalcin; OPN: Osteopontin. *P < 0.05, **P < 0.01 vs PCL-B traditional scaffold. n=3, x±s |
3 讨论
理想的骨修复支架除了具有良好的组织相容性和合理的骨生长空间之外,还应具有一定的骨诱导性[1-2]。BMP是转化生长因子家族的一员,是公认的最重要的骨组织工程细胞因子之一,其强大的骨诱导活性已被大量实验证实[4-5]。高剂量BMP的成骨诱导效应虽好,但是成骨速度过快会导致成骨质量低和骨吸收,而小剂量成骨诱导效应欠佳[8]。此外,BMP在体内的半衰期很短,难以长时间发挥作用[9-11]。因此,探索BMP的长时间缓释方法具有重要意义。
针对负载BMP-2并在支架中长时间缓慢释放这一目标,以及改良已有高分子材料的负载蛋白能力以达到保护BMP-2在制备过程中不被损伤的目的,并希望能取得良好的细胞成骨诱导效应,已有大量研究尝试将BMP-2负载在不同的生物材料载体中,构建的部分生物材料支架已经应用于动物实验甚至临床治疗,但是治疗效果均不理想[12-13],其主要原因是BMP-2极易失活。而本研究在制备的PCL-B/P复合支架上种植人源性BMSC后,检测其增殖能力,结果显示PCL-B/P复合支架上BMSC的增殖能力优于PCL-B传统支架,说明PCL-B/P复合支架负载及缓释的BMP-2活性良好,有利于BMSC的增殖生长。
本研究利用PL的两亲性将亲水的BMP-2改性为可在二氯甲烷中分散的疏水B/P,然后将B/P和PCL材料混合使BMP-2均匀地分散在PCL内部,所制备的三维PCL-B/P复合支架也可负载BMP-2。与PCL-B传统支架相比,PCL-B/P复合支架的缓释效果可长达22 d。同时,通过SEM观察发现PCL-B/P复合支架与PCL-B传统支架的结构相似,均为多孔海绵状,且平均孔径也非常接近,说明加入了PL的PCL-B/P复合支架的多孔结构并无明显改变。
既往研究中, 负载BMP-2的复合支架多是将未加修饰的BMP-2直接加入高分子材料,BMP的亲水性和高分子材料的疏水性使两者混合后易分离,无法实现均匀分散。Lin等[12]尝试将BMP-2直接混合在疏水性的聚乳酸-羟基乙酸共聚物 (PLGA) 中得到多孔海绵状支架,在释放测试的第7天BMP-2的释放就已经达到平衡,释放时间远短于本研究的22 d。也有学者尝试用无机材料混合改性高分子材料,使其能一定程度上吸附BMP。Rai等[13]利用3D生物打印技术制备出可负载BMP-2的聚己内酯-磷酸三钙 (PCL-TCP) 支架,虽然BMP的释放能持续21 d,且持续释放量已经高达95%~100%,远高于本研究中的80%;但TCP易堵塞3D打印设备,当TCP含量过多时PCL-TCP支架脆弱,无法应用于临床治疗。因此,本研究制备的PCL-B/P复合支架缓释效果佳,缓释时间长,有很大的临床应用潜力。
ALP是BMSC成骨分化的生物标记物之一,可用于判断BMSC的成骨分化能力[14]。单位细胞内的ALP含量越高,说明细胞成骨分化能力越强,也间接证实所处环境中BMP-2的含量越多[15-16]。此外,COL-Ⅰ[17-19]、OPN[20]和OC[21-23]等基因也参与细胞矿化,其表达水平也是判断BMSC成骨分化的重要依据。在本研究制备的PCL-B/P复合支架上种植人源性BMSC,检测细胞的ALP活性和COL-Ⅰ、OPN、OC mRNA的表达水平,结果显示PCL-B/P复合支架上BMSC的成骨分化能力优于PCL-B传统支架,说明PCL-B/P复合支架上可负载且缓慢释放BMP-2的微环境更有利于BMSC的成骨分化。
综上所述,本文采用PL修饰的方法,促进BMP-2在PCL的分散和包裹,成功制备出一种可负载且长时间缓慢释放BMP-2的PCL-B/P复合支架,且成功实现了人源性BMSC向成骨的分化,其细胞增殖能力和成骨分化能力都优于PCL-B传统支架,具有潜在的骨组织工程应用价值。
| [1] | BIGHAM-SADEGH A, ORYAN A. Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering[J]. Connect Tissue Res, 2015, 56: 175–194. DOI: 10.3109/03008207.2015.1027341 |
| [2] | BRUNELLO G, SIVOLELLA S, MENEGHELLO R, FERRONI L, GARDIN C, PIATTELLI A, et al. Powder-based 3D printing for bone tissue engineering[J]. Biotechnol Adv, 2016, 34: 740–753. DOI: 10.1016/j.biotechadv.2016.03.009 |
| [3] | FERNANDEZ J M, MOLINUEVO M S, CORTIZO M S, CORTIZO A M. Development of an osteoconductive PCL-PDIPF-hydroxyapatite composite scaffold for bone tissue engineering[J]. J Tissue Eng Regen Med, 2011, 5: e126–e135. DOI: 10.1002/term.394 |
| [4] | BESSA P C, CASAL M, REIS R L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part Ⅱ (BMP delivery)[J]. J Tissue Eng Regen Med, 2008, 2: 81–96. DOI: 10.1002/(ISSN)1932-7005 |
| [5] | KUBOKI Y, SASAKI M, SAITO A, TAKITA H, KATO H. Regeneration of periodontal ligament and cementum by BMP-applied tissue engineering[J]. Eur J Oral Sci, 1998, 106(Suppl 1): 197–203. |
| [6] | YAO Q, WEI B, GUO Y, JIN C, DU X, YAN C, et al. Design, construction and mechanical testing of digital 3D anatomical data-based PCL-HA bone tissue engineering scaffold[J]. J Mater Sci Mater Med, 2015, 26: 5360. DOI: 10.1007/s10854-015-3080-6 |
| [7] | PENG Q, ZHANG Z R, GONG T, CHEN G Q, SUN X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles[J]. Biomaterials, 2012, 33: 1583–1588. DOI: 10.1016/j.biomaterials.2011.10.072 |
| [8] | PAULY S, KLATTE F, STROBEL C, SCHMIDMAIER G, GREINER S, SCHEIBEL M, et al. BMP-2 and BMP-7 affect human rotator cuff tendon cells in vitro[J]. J Shoulder Elbow Surg, 2012, 21: 464–473. DOI: 10.1016/j.jse.2011.01.015 |
| [9] | YILGOR P, HASIRCI N, HASIRCI V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules[J]. J Biomed Mater Res A, 2010, 93: 528–536. |
| [10] | MACIAS D, GANAN Y, SAMPATH T K, PIEDRA M E, ROS M A, HURLE J M. Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development[J]. Development, 1997, 124: 1109–1117. |
| [11] | LYONS K M, HOGAN B L, ROBERTSON E J. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development[J]. Mech Dev, 1995, 50: 71–83. DOI: 10.1016/0925-4773(94)00326-I |
| [12] | LIN Z Y, DUAN Z X, GUO X D, LI J F, LU H W, ZHENG Q X, et al. Bone induction by biomimetic PLGA-(PEG-ASP) n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo[J]. J Control Release, 2010, 144: 190–195. DOI: 10.1016/j.jconrel.2010.02.016 |
| [13] | RAI B, TEOH S H, HUTMACHER D W, CAO T, HO K H. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2[J]. Biomaterials, 2005, 26: 3739–3748. DOI: 10.1016/j.biomaterials.2004.09.052 |
| [14] | ZHAO K W, MURRAY S S, MURRAY E J. Secreted phosphoprotein-24 kDa (Spp24) attenuates BMP-2-stimulated Smad 1/5 phosphorylation and alkaline phosphatase induction and was purified in a protective complex with α2-macroglobulins from serum[J]. J Cell Biochem, 2013, 114: 378–387. DOI: 10.1002/jcb.v114.2 |
| [15] | WANG X, OYANE A, TSURUSHIMA H, SOGO Y, LI X, ITO A. BMP-2 and ALP gene expression induced by a BMP-2 gene-fibronectin-apatite composite layer[J]. Biomed Mater, 2011, 6: 045004. DOI: 10.1088/1748-6041/6/4/045004 |
| [16] | MÖLDERS M, FELIX J, BINGMANN D, HIRNER A, WIEMANN M. Uptake of nickel from 316L stainless steel into contacting osteoblastic cells and metal ion interference with BMP-2-induced alkaline phosphatase[J]. J Biomed Mater Res A, 2007, 83: 303–312. |
| [17] | RAWADI G, VAYSSIERE B, DUNN F, BARON R, ROMAN-ROMAN S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop[J]. J Bone Miner Res, 2003, 18: 1842–1853. DOI: 10.1359/jbmr.2003.18.10.1842 |
| [18] | RASI GHAEMI S, DELALAT B, CETÓ X, HARDING F J, TUKE J, VOELCKER N H. Synergistic influence of collagen Ⅰ and BMP 2 drives osteogenic differentiation of mesenchymal stem cells: a cell microarray analysis[J]. Acta Biomater, 2016, 34: 41–52. DOI: 10.1016/j.actbio.2015.07.027 |
| [19] | SUMANASINGHE R D, BERNACKI S H, LOBOA E G. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression[J]. Tissue Eng, 2006, 12: 3459–3465. DOI: 10.1089/ten.2006.12.3459 |
| [20] | HE X, YANG X, JABBARI E. Combined effect of osteopontin and BMP-2 derived peptides grafted to an adhesive hydrogel on osteogenic and vasculogenic differentiation of marrow stromal cells[J]. Langmuir, 2012, 28: 5387–5397. DOI: 10.1021/la205005h |
| [21] | MACHADO C, CORREA C, MEDRADO G, LEITE M, GOES A. Ectopic expression of telomerase enhances osteopontin and osteocalcin expression during osteogenic differentiation of human mesenchymal stem cells from elder donors[J]. J Stem Cells Regen Med, 2009, 5: 49–57. |
| [22] | NAKAMURA A, DOHI Y, AKAHANE M, OHGUSHI H, NAKAJIMA H, FUNAOKA H, et al. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells[J]. Tissue Eng Part C Methods, 2009, 15: 169–180. DOI: 10.1089/ten.tec.2007.0334 |
| [23] | CAO T, HENG B C, YE C P, LIU H, TOH W S, ROBSON P, et al. Osteogenic differentiation within intact human embryoid bodies result in a marked increase in osteocalcin secretion after 12 days of in vitro culture, and formation of morphologically distinct nodule-like structures[J]. Tissue Cell, 2005, 37: 325–334. DOI: 10.1016/j.tice.2005.03.008 |
 2017, Vol. 38
2017, Vol. 38


