人体肾脏每日通过血液超滤产生约180 L原尿,原尿经由近端小管、髓襻降支、髓襻升支、远端小管及集合管进入膀胱,在这一过程中,尿液的容量及组成被改变,最终只有约1%的原尿形成终尿排出。肾小管及集合管管腔上分布的水通道蛋白 (aquaporin, AQP) 在细胞膜上组成“孔道”,控制水的进出,使肾单位可以从快速经过的原尿中进行水的重吸收[1]。目前在哺乳动物体内已发现13种AQP (AQP0~AQP12),其中有9种 (AQP1~8和AQP11) 表达于肾脏中[2-4]。AQP5、AQP6表达于连接小管及集合管闰细胞中,其中AQP5作用未明,AQP6主要与尿酸分泌及硝酸盐和氯化物的转运有关[5-6]; AQP7、AQP8表达于近端小管,主要负责甘油代谢与尿素、氨及活性氧的转运[7-9];AQP11同样表达于近端小管,但并未参与尿液的形成过程,可能与内质网稳态相关,其缺失将导致多囊性肾病[10]。在尿液形成过程中,发挥水的重吸收作用的主要是AQP1~4。
AQP1表达于近端小管与髓襻降支细段,负责约90%的水的重吸收,这种重吸收方式是由渗透压驱动的被动重吸收,不受神经体液因素调节。剩余的水的重吸收则是由集合管主细胞通过主动重吸收的方式进行调控,受神经体液因素调节,而这种主动重吸收则依赖于表达于其中的AQP。集合管主细胞表达3种AQP:AQP2、AQP3和AQP4。AQP3与AQP4分布于主细胞基底膜侧,其功能是将主细胞重吸收的水分经由基底膜转运至血液[11-12],而对于原尿的水的重吸收则依靠AQP2。AQP2表达于主细胞管腔侧质膜及胞内囊泡内。在去除精氨酸加压素 (arginine vasopressin,AVP) 的情况下,AQP2主要表达于胞内囊泡内,此时主细胞管腔侧质膜对水的重吸收能力低下;当AVP与表达于基底膜上的G蛋白偶联受体V2R结合时,环磷酸腺苷/蛋白激酶A (cyclic adenosine monophosphate/protein kinase A, cAMP/PKA) 信号通路被激活,导致AQP2由胞内囊泡转移至顶端质膜,大大增加了主细胞对水的重吸收能力[13]。AQP2的这种再分布的调控方式被称为AQP2的短时调控,又称为囊泡穿梭机制 (图 1)。磷酸化、蛋白间相互作用、细胞骨架、钙离子等多种因素参与了调控AQP2的这一转移过程,本文就AQP2囊泡穿梭机制的研究进展作一综述。
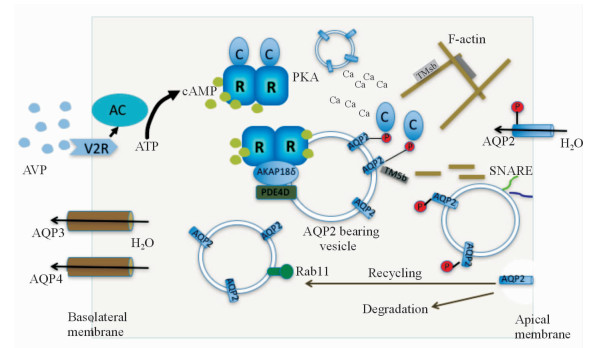
|
图 1 AVP介导的集合管主细胞AQP2囊泡穿梭模型 Fig 1 Model of the AVP-stimulated AQP2 translocation from intracellular vesicles into the plasma membrane of renal collecting duct principal cells AVP: Arginine vasopressin; AQP: Aquaporin; AC: Adenylate cyclase; V2R: Vasopressin type 2 receptor; ATP: Adenosine triphosphate; cAMP: Cyclic adenosine monophosphate; PKA: Protein kinase A; AKAP: A-kinase anchoring protein; SNARE: Soluble NSF attachment protein receptor; PDE: Phosphodiesterase; C: C subunit; R: R subunit; P: Phosphoric acid |
1 磷酸化
AQP2是第1个被发现的可由外界刺激因素改变其细胞内定位的AQP,cAMP依赖的PKA激活AQP2羧基末端丝氨酸第256位点 (S256) 的磷酸化在这个过程中起关键作用[14]。通过基因突变分析发现,模拟磷酸化的AQP2 S256位点的突变形式 (AQP2-S256D) 持续分布于集合管主细胞顶端质膜,而非磷酸化的AQP2(AQP2-S256A) 在cAMP的持续刺激下仍不能转移至顶端质膜[15]。其他激酶例如环磷酸鸟苷 (cyclic guanosine monophosphate, cGMP) 依赖的蛋白激酶G (protein kinase G, PKG) 同样可以引起AQP2-S256的磷酸化,进而导致AQP2转移至顶端质膜,其激活因素包括一氧化氮、利钠肽、西地那非等[16]。Procino等[17]发现高尔基体酪蛋白激酶 (G-CK) 对AQP2的磷酸化作用参与了AQP2由高尔基体转移至囊泡的过程以及后续过程,AQP2-S256A并非G-CK的底物而被转移至溶酶体,提示AQP2 S256位点的磷酸化可能与AQP2的成熟及其由内质网转移至高尔基体有关。
除S256外,S261、S264、S269也是AQP2的磷酸化位点,同样可由AVP调节。S261位点平静状态下AQP2保持磷酸化状态位于胞内,在AVP刺激后可引起其去磷酸化[18],其机制可能是通过加快AQP2的降解来调节AQP2的内吞作用[19]。S264与S269位点磷酸化的先决条件是S256位点的磷酸化[20],其作用可能是增加AQP2在顶端质膜的滞留能力,但具体机制尚不明确。
2 激酶锚定蛋白 (A-kinase anchoring protein,AKAP) 和磷酸二酯酶 (phosphodiesterase,PDE)AQP2在细胞内并非自由移动,而是在精确的细胞内位点通过蛋白间相互作用来进行移动的,AKAP作为一种骨架蛋白,其作用是使PKA精确地作用于其底物[21]。使用AKAP-Lbc和AKAP18δ的PKA连接域来源的多肽预处理小鼠肾脏内髓集合管 (inner medullary collecting duct,IMCD) 细胞可发现AVP诱导的AQP2穿梭转移被打断[22-23], 揭示了AKAP使PKA精确作用于AQP2是AQP2穿梭的先决条件,同样也可以解释AVP增加核周PKA活性的原因[24]——AQP2囊泡在安静状态下主要位于核周。目前已发现数种AKAP表达于AQP2的囊泡,包括前述的AKAP-Lbc、AKAP18δ,以及AKAP220[25]和Jo等[26]发现的一种相对分子质量为90 000的AKAP。除PKA外,AKAP还可以连接蛋白激酶Cζ (protein kinase Cζ, PKCζ)、蛋白磷酸酶2B (protein phosphatase 2B, PP2B)、PDE等[24, 26-27]。
PDE对cAMP的水解作用是cAMP信号通路的终点,在PDE的11种同工酶家族中,PDE4家族特异性地调节cAMP的活性,进而调节PKA的活性[28-29]。在AQP2囊泡中,AKAP18δ与PDE4D直接作用,阻止安静状态下AQP2插入IMCD顶端质膜,进而增加水的重吸收,PDE4抑制剂咯利普兰 (rolipram) 可以逆转这种作用[22,24]。
3 细胞骨架越来越多的证据表明细胞骨架的动态变化在AQP2的细胞内再分布过程中起着关键作用。AQP2在内质网中折叠并聚集为同源四聚体,然后穿过高尔基体贮存在核周的细胞内囊泡内。在基础状态下, AQP2与球状肌动蛋白结合, 原肌球蛋白5b与F-肌动蛋白结合以维持F-肌动蛋白网的稳定, 阻止AQP2转运。AQP2 S256磷酸化后从球状肌动蛋白中解离, 并与原肌球蛋白5b结合, 原肌球蛋白5b从F-肌动蛋白中解离, F-肌动蛋白网部分解聚, 使囊泡可以转运到质膜[30]。此外,也有证据表明微管的重组同样参与了AQP2的穿梭,外源性给予破坏细胞微管的诺考达唑 (nocodazole) 和秋水仙胺 (denecolcine) 可以阻碍AVP介导的水的重吸收[31],但具体机制目前尚存在争议。小分子质量的GTP蛋白Rho家族也参与了肌动蛋白的动态调控[32],RhoA的激活导致F-激动蛋白张力纤维的形成并阻碍AQP2的转移,而PKA磷酸化通路则通过阻碍RhoA的激活介导肌动蛋白的解聚。在体外培养的肾脏集合管细胞CD8细胞中给予磷酸酶1和2a的抑制剂冈田酸 (okadaic acid),可以观察到AQP2-S256磷酸化增加了60%并导致肌动蛋白网的解聚[33]。这些发现同时证明了AQP2的再分布过程不仅仅受到PKA的调控。
4 钙离子肾脏集合管细胞在AVP的刺激下,除了导致cAMP浓度升高外,尚能升高细胞内Ca2+的浓度[34]。AVP导致集合管细胞内Ca2+动员并非是通过经典的G蛋白Gq,也非是通过磷酸肌醇的水解作用实现,目前认为其作用机制可能是通过Gs蛋白激活腺苷酸环化酶的下游效应。并且,此效应并非必需PKA参加,而是通过cAMP的另一个交换蛋白的效应器Epac来实现。利阿诺定 (ryanodine) 可以抑制此效应,提示细胞内动员的Ca2+可以激活肌浆网利阿诺定受体进而引起细胞内钙库释放,导致Ca2+浓度增加[35]。Ca2+浓度增加可以进一步激活肌球蛋白轻链激酶 (calcium/calmodulin-dependent myosin light chain kinase, MLCK),进而促进AQP2囊泡转运[36],增加集合管细胞的水的重吸收能力。近期,Ranieri等[37]描述并验证了一个新的现象,细胞外高浓度的Ca2+会通过Ca2+敏感受体负反馈性地减弱AVP介导的AQP2的囊泡穿梭,提示机体Ca2+深度参与了机体水平衡的调节。
5 SNARESNARE最初是作为N-乙基马来酰胺-敏感因子 (N-ethyl-maleimide-sensitive factor, NSF) 和可溶性NSF附着蛋白 (soluble NSF attachment protein, SNAP) 的膜受体,即SNAP蛋白受体 (SNAP receptor, SNARE),其命名正源于此。根据其分布和功能, SNARE被分为t-SNARE (target membrane SNARE) 和v-SNARE (vesicle membrane SNARE)。有证据表明t-SNARE可在膜上形成稳定的子复合体并引导v-SNARE与其结合形成稳定的SNARE复合体[38]。t-SNARE包括syntaxin和SNAP家族,v-SNARE包括VAMP/synaptobrevin及其相关蛋白。AQP2经由胞吐的形式嵌入细胞膜的过程同样涉及SNARE。AQP2囊泡表达VAMP2和VAMP3,与膜上表达的SNAP23和syntaxin3相互作用并融合,使AQP2嵌入细胞质膜上[39]。Wang等[40]发现,VAMP8敲除的小鼠严重损害AQP2囊泡的外分泌,进一步研究发现VAMP8表达于AQP2囊泡并与质膜上的syntaxin3和syntaxin4相互作用,以调节AQP2囊泡与质膜的融合。是否有更多的SNAREs参与AQP2的穿梭调节仍需进一步的研究。
6 胞吞作用机体对AQP2在细胞膜上丰度的调控不仅仅体现在对AQP2囊泡胞吐过程的调节,内吞作用同样调节AQP2在集合管主细胞管腔侧质膜的表达并受到机体调控。网格蛋白介导的内吞作用是目前文献报道的最多的调控方式之一。AQP2通过介导水的重吸收调整机体水平衡并导致机体AVP分泌减少,进而导致AQP2的内在化。AQP2与网格蛋白小窝结合, 通过网格蛋白途径内吞到表达早期内吞体相关蛋白1的早期小体内, 随后转移到表达Rab11 (一种单体GTP酶) 的特异AQP2储存囊泡内[41-42]。热休克蛋白70 (Hsp70) 也参与了AQP2内在化的过程,其功能与网格蛋白包被小泡的去包被有关[43]。AVP诱导AQP2磷酸化的同时抑制了它与Hsp70的结合, 促进胞吐的同时抑制其内吞[44]。Kamsteeg等[45]发现膜磷脂与淋巴细胞相关蛋白 (myelin and lymphocyte-associated protein, MAL) 也参与AQP2的穿梭转移,MAL位于主细胞的管腔质膜侧,AQP2 S256位点的磷酸化可以加强其与MAL的相互作用,阻碍AQP2的内在化,这同时也解释了pS256-AQP2在质膜上稳定聚集的现象。
独立于AQP2的磷酸化或去磷酸化之外,PKC的激活同样可以调节AQP2的内在化过程。PKC的激活可以加速AQP2的内在化[15]并引导AQP2进入溶酶体[46]。其机制与AQP2赖氨酸第270位点的泛素化有关。然而令人惊奇的是,血管紧张素Ⅱ与其受体AT1结合后PKC依赖的途径增加了cAMP的水平并增强了AVP介导的AQP2的囊泡穿梭[47],其具体机制尚不明确。
7 小结与展望AQP2在集合管主细胞的定位对于机体水的重吸收至关重要。AQP2若位于主细胞管腔侧质膜将会促进原尿的水的重吸收。对于心力衰竭、抗利尿激素分泌异常综合征 (syndrome of inappropriate antidiuretic hormone secretion, SIADH) 和肝硬化患者,管腔侧质膜AQP2的过表达会导致过多的水潴留。相反,细胞内囊泡AQP2占优势的尿崩症患者则以大量的低渗尿为特征。cAMP依赖和非cAMP依赖的多种通路以及蛋白间的相互作用参与了AQP2定位的调控。不论是细胞质膜AQP2过表达还是低表达,对于它们的医疗需求还远远没有满足,也为以AQP2穿梭机制作为药理学靶点的新药研究提供了广阔的前景。目前已经在动物及部分人体试验中验证了他汀类药物可以促进AQP2的囊泡穿梭[48],而V2受体拮抗剂及非甾体类的抗炎药可以抑制AQP2向质膜转移[49]。然而它们带来的不良反应也为它们的广泛应用带来了限制。因而仍有必要继续阐明AQP2囊泡穿梭背后的分子机制,进而为新药的开发提供靶点。蛋白间相互作用的高度特异性和多样性使其可能成为一种新药的作用靶点。
| [1] | AGRE P. The aquaporin water channels[J]. Proc Am Thorac Soc, 2006, 3: 5–13. DOI: 10.1513/pats.200510-109JH |
| [2] | KNEPPER M A, KWON T H, NIELSEN S. Molecular physiology of water balance[J]. N Engl J Med, 2015, 372: 1349–1358. DOI: 10.1056/NEJMra1404726 |
| [3] | KWON T H, NIELSEN J, MØLLER H B, FENTON R A, NIELSEN S, FRØKIAER J. Aquaporins in the kidney[J]. Handb Exp Pharmacol, 2009, 190: 95–132. DOI: 10.1007/978-3-540-79885-9 |
| [4] | PROCINO G, MASTROFRANCESCO L, SALLUSTIO F, COSTANTINO V, BARBIERI C, PISANI F, et al. AQP5 is expressed in type-B intercalated cells in the collecting duct system of the rat, mouse and human kidney[J]. Cell Physiol Biochem, 2011, 28: 683–692. DOI: 10.1159/000335762 |
| [5] | WU H, CHEN L, ZHANG X, ZHOU Q, LI J M, BERGER S, et al. Aqp5 is a new transcriptional target of Dot1a and a regulator of Aqp2[J]. PLoS One, 2013, 8: e53342. DOI: 10.1371/journal.pone.0053342 |
| [6] | YASUI M. pH regulated anion permeability of aquaporin-6[J]. Handb Exp Pharmacol, 2009, 190: 299–308. DOI: 10.1007/978-3-540-79885-9 |
| [7] | SOHARA E, RAI T, SASAKI S, UCHIDA S. Physiological roles of AQP7 in the kidney: lessons from AQP7 knockout mice[J]. Biochim Biophys Acta, 2006, 1758: 1106–1110. DOI: 10.1016/j.bbamem.2006.04.002 |
| [8] | TAMMA G, PROCINO G, SVELTO M, VALENTI G. Cell culture models and animal models for studying the patho-physiological role of renal aquaporins[J]. Cell Mol Life Sci, 2012, 69: 1931–1946. DOI: 10.1007/s00018-011-0903-3 |
| [9] | SAPAROV S M, LIU K, AGRE P, POHL P. Fast and selective ammonia transport by aquaporin-8[J]. J Biol Chem, 2007, 282: 5296–5301. DOI: 10.1074/jbc.M609343200 |
| [10] | MORISHITA Y, MATSUZAKI T, HARA-CHIKUMA M, ANDOO A, SHIMONO M, MATSUKI A, et al. Disruption of aquaporin-11 produces polycystic kidneys following vacuolization of the proximal tubule[J]. Mol Cell Biol, 2005, 25: 7770–7779. DOI: 10.1128/MCB.25.17.7770-7779.2005 |
| [11] | TERRIS J, ECELBARGER C A, MARPLES D, KNEPPER M A, NIELSEN S. Distribution of aquaporin-4 water channel expression within rat kidney[J]. Am J Physiol, 1995, 269(6 Pt 2): F775–F785. |
| [12] | ECELBARGER C A, TERRIS J, FRINDT G, ECHEVARRIA M, MARPLES D, NIELSEN S, et al. Aquaporin-3 water channel localization and regulation in rat kidney[J]. Am J Physiol, 1995, 269(5 Pt 2): F663–F672. |
| [13] | NIELSEN S, CHOU C L, MARPLES D, CHRISTENSEN E I, KISHORE B K, KNEPPER M A. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane[J]. Proc Natl Acad Sci USA, 1995, 92: 1013–1017. DOI: 10.1073/pnas.92.4.1013 |
| [14] | ARTHUR J, HUANG J, NOMURA N, JIN W W, LI W, CHENG X, et al. Characterization of the putative phosphorylation sites of the AQP2 C terminus and their role in AQP2 trafficking in LLC-PK1 cells[J]. Am J Physiol Renal Physiol, 2015, 309: F673–F679. DOI: 10.1152/ajprenal.00152.2015 |
| [15] | VAN BALKOM B W, SAVELKOUL P J, MARKOVICH D, HOFMAN E, NIELSEN S, VAN DER SLUIJS P, et al. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel[J]. J Biol Chem, 2002, 277: 41473–41479. DOI: 10.1074/jbc.M207525200 |
| [16] | BOULEY R, BRETON S, SUN T, MCLAUGHLIN M, NSUMU N N, LIN H Y, et al. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells[J]. J Clin Invest, 2000, 106: 1115–1126. DOI: 10.1172/JCI9594 |
| [17] | PROCINO G, CARMOSINO M, MARIN O, BRUNATI A M, CONTRI A, PINNA L A, et al. Ser-256 phosphorylation dynamics of aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells[J]. FASEB J, 2003, 17: 1886–1888. |
| [18] | HOFFERT J D, NIELSEN J, YU M J, PISITKUN T, SCHLEICHER S M, NIELSEN S, et al. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct[J]. Am J Physiol Renal Physiol, 2007, 292: F691–F700. |
| [19] | TAMMA G, ROBBEN J H, TRIMPERT C, BOONE M, DEEN P M. Regulation of AQP2 localization by S256 and S261 phosphorylation and ubiquitination[J]. Am J Physiol Cell Physiol, 2011, 300: C636–C646. DOI: 10.1152/ajpcell.00433.2009 |
| [20] | MOELLER H B, MACAULAY N, KNEPPER M A, FENTON R A. Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: evidence against channel gating[J]. Am J Physiol Renal Physiol, 2009, 296: F649–F657. |
| [21] | LANGEBERG L K, SCOTT J D. Signalling scaffolds and local organization of cellular behaviour[J]. Nat Rev Mol Cell Biol, 2015, 16: 232–244. DOI: 10.1038/nrm3966 |
| [22] | SZASZÁK M, CHRISTIAN F, ROSENTHAL W, KLUSSMANN E. Compartmentalized cAMP signalling in regulated exocytic processes in non-neuronal cells[J]. Cell Signal, 2008, 20: 590–601. DOI: 10.1016/j.cellsig.2007.10.020 |
| [23] | KLUSSMANN E, MARIC K, WIESNER B, BEYERMANN M, ROSENTHAL W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells[J]. J Biol Chem, 1999, 274: 4934–4938. DOI: 10.1074/jbc.274.8.4934 |
| [24] | STEFAN E, WIESNER B, BAILLIE G S, MOLLAJEW R, HENN V, LORENZ D, et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells[J]. J Am Soc Nephrol, 2007, 18: 199–212. DOI: 10.1681/ASN.2006020132 |
| [25] | OKUTSU R, RAI T, KIKUCHI A, OHNO M, UCHIDA K, SASAKI S, et al. AKAP220 colocalizes with AQP2 in the inner medullary collecting ducts[J]. Kidney Int, 2008, 74: 1429–1433. DOI: 10.1038/ki.2008.402 |
| [26] | JO I, WARD D T, BAUM M A, SCOTT J D, COGHLAN V M, HAMMOND T G, et al. AQP2 is a substrate for endogenous PP2B activity within an inner medullary AKAP-signaling complex[J]. Am J Physiol Renal Physiol, 2001, 281: F958–F965. |
| [27] | McSORLEY T, STEFAN E, HENN V, WIESNER B, BAILLIE G S, HOUSLAY M D, et al. Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells[J]. Eur J Cell Biol, 2006, 85: 673–678. DOI: 10.1016/j.ejcb.2006.01.005 |
| [28] | MIKA D, CONTI M. PDE4D phosphorylation: A coincidence detector integrating multiple signaling pathways[J]. Cell Signal, 2016, 28: 719–724. DOI: 10.1016/j.cellsig.2015.11.001 |
| [29] | KLUSSMANN E. Protein-protein interactions of PDE4 family members-Functions, interactions and therapeutic value[J]. Cell Signal, 2016, 28: 713–718. DOI: 10.1016/j.cellsig.2015.10.005 |
| [30] | NODA Y, HORIKAWA S, KANDA E, YAMASHITA M, MENG H, ETO K, et al. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking[J]. J Cell Biol, 2008, 182: 587–601. DOI: 10.1083/jcb.200709177 |
| [31] | VOSSENKÄMPER A, NEDVETSKY P I, WIESNER B, FURKERT J, ROSENTHAL W, KLUSSMANN E. Microtubules are needed for the perinuclear positioning of aquaporin-2 after its endocytic retrieval in renal principal cells[J]. Am J Physiol Cell Physiol, 2007, 293: C1129–C1138. DOI: 10.1152/ajpcell.00628.2006 |
| [32] | LOO C S, CHEN C W, WANG P J, CHEN P Y, LIN S Y, KHOO K H, et al. Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells[J]. Proc Natl Acad Sci USA, 2013, 110: 17119–17124. DOI: 10.1073/pnas.1309219110 |
| [33] | VALENTI G, PROCINO G, CARMOSINO M, FRIGERI A, MANNUCCI R, NICOLETTI I, et al. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells[J]. J Cell Sci, 2000, 113(Pt 11): 1985–1992. |
| [34] | STAR R A, NONOGUCHI H, BALABAN R, KNEPPER M A. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct[J]. J Clin Invest, 1988, 81: 1879–1888. DOI: 10.1172/JCI113534 |
| [35] | YIP K P. Epac-mediated Ca2+ mobilization and exocytosis in inner medullary collecting duct[J]. Am J Physiol Renal Physiol, 2006, 291: F882–F890. DOI: 10.1152/ajprenal.00411.2005 |
| [36] | CHOU C L, CHRISTENSEN B M, FRISCHE S, VORUM H, DESAI R A, HOFFERT J D, et al. Non-muscle myosin Ⅱ and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct[J]. J Biol Chem, 2004, 279: 49026–49035. DOI: 10.1074/jbc.M408565200 |
| [37] | RANIERI M, TAMMA G, DI MISE A, RUSSO A, CENTRONE M, SVELTO M, et al. Negative feedback from CaSR signaling to aquaporin-2 sensitizes vasopressin to extracellular Ca2+[J]. J Cell Sci, 2015, 128: 2350–2360. DOI: 10.1242/jcs.168096 |
| [38] | MALSAM J, S LLNER T H. Organization of SNAREs within the Golgi stack[J]. Cold Spring Harb Perspect Biol, 2011, 3: a005249. DOI: 10.1101/cshperspect.a005249 |
| [39] | PROCINO G, BARBIERI C, TAMMA G, DE BENEDICTIS L, PESSIN J E, SVELTO M, et al. AQP2 exocytosis in the renal collecting duct-involvement of SNARE isoforms and the regulatory role of Munc18b[J]. J Cell Sci, 2008, 121(Pt 12): 2097–2106. |
| [40] | WANG C C, NG C P, SHI H, LIEW H C, GUO K, ZENG Q, et al. A role for VAMP8/endobrevin in surface deployment of the water channel aquaporin 2[J]. Mol Cell Biol, 2010, 30: 333–343. DOI: 10.1128/MCB.00814-09 |
| [41] | SUN T X, VAN HOEK A, HUANG Y, BOULEY R, McLAUGHLIN M, BROWN D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin[J]. Am J Physiol Renal Physiol, 2002, 282: F998–F1011. DOI: 10.1152/ajprenal.00257.2001 |
| [42] | NEDVETSKY P I, STEFAN E, FRISCHE S, SANTAMARIA K, WIESNER B, VALENTI G, et al. A Role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle[J]. Traffic, 2007, 8: 110–123. DOI: 10.1111/j.1600-0854.2006.00508.x |
| [43] | CHANG H C, NEWMYER S L, HULL M J, EBERSOLD M, SCHMID S L, MELLMAN I. Hsc70 is required for endocytosis and clathrin function in Drosophila[J]. J Cell Biol, 2002, 159: 477–487. DOI: 10.1083/jcb.200205086 |
| [44] | MOELLER H B, PRAETORIUS J, R TZLER M R, FENTON R A. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions[J]. Proc Natl Acad Sci USA, 2010, 107: 424–429. DOI: 10.1073/pnas.0910683107 |
| [45] | KAMSTEEG E J, DUFFIELD A S, KONINGS I B, SPENCER J, PAGEL P, DEEN P M, et al. MAL decreases the internalization of the aquaporin-2 water channel[J]. Proc Natl Acad Sci USA, 2007, 104: 16696–16701. DOI: 10.1073/pnas.0708023104 |
| [46] | KAMSTEEG E J, HENDRIKS G, BOONE M, KONINGS I B, OORSCHOT V, VAN DER SLUIJS P, et al. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel[J]. Proc Natl Acad Sci USA, 2006, 103: 18344–18349. DOI: 10.1073/pnas.0604073103 |
| [47] | LEE Y J, SONG I K, JANG K J, NIELSEN J, FRØKIAER J, NIELSEN S, et al. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin Ⅱ through AT1 receptor[J]. Am J Physiol Renal Physiol, 2007, 292: F340–F350. |
| [48] | BONFRATE L, PROCINO G, WANG D Q, SVELTO M, PORTINCASA P. A novel therapeutic effect of statins on nephrogenic diabetes insipidus[J]. J Cell Mol Med, 2015, 19: 265–282. DOI: 10.1111/jcmm.2015.19.issue-2 |
| [49] | REN H, YANG B, MOLINA P A, SANDS J M, KLEIN J D. NSAIDs alter phosphorylated forms of AQP2 in the inner medullary tip[J/OL]. PLoS One, 2015, 10: e0141714. doi: 10.1371/journal.pone.0141714.eCollection 2015. |
 2017, Vol. 38
2017, Vol. 38


