2. 第二军医大学学员旅学员3队, 上海 200433;
3. 第二军医大学国际交流学院, 上海 200433
2. The Third Student Team, Student Brigade, Second Military Medical University, Shanghai 200433, China;
3. College of International Exchange, Second Military Medical University, Shanghai 200433, China
慢性应激对机体代谢和免疫有着广泛的影响,与多种慢性疾病的发生、发展密切相关[1]。研究发现慢性应激能够诱发小鼠产生非酒精性脂肪肝(non-alcoholic fatty liver disease,NAFLD)[2],但其机制尚不明确。
磷酸腺苷激活的蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK)是调节能量代谢的关键酶,对机体炎性反应和脂代谢具有重要的调节作用[3-4],而炎症和脂代谢紊乱与NAFLD的发生密切相关[5-6],提示AMPK可能在慢性应激导致NAFLD的过程中发挥作用。因此,本实验探讨了AMPK及其激动剂在慢性应激诱发NAFLD中的作用及机制。
1 材料和方法 1.1 小鼠慢性应激模型的制备雄性BABL/c小鼠,体质量(20±2) g,由第二军医大学实验动物中心提供[生产许可证号:SCXK(沪)2013-0016]。每天在不固定时间点随机采用一种应激方法干预小鼠,共有7种应激方法可选,分别为束缚应激2 h、垫料打湿24 h、摇晃10 min、鼠笼倾斜24 h、饮食剥夺2 h、45 ℃烘箱热应激5 min、4 ℃水游泳冷应激5 min。所有小鼠均连续应激干预5周[7]。
1.2 AMPK激动剂5-氨基咪唑-4-甲酰胺核苷酸(5-aminoimidazole-4-carboxamide ribonucleoside,AICAR,美国Sigma公司),剂量为500 mg/kg体质量,每次应激前1 h小鼠腹腔内注射。
1.3 小鼠实验分组实验鼠分为4组,分别为对照组(Con组)、应激组(ST组)、应激加AICAR给药组(ST+AICAR组)和AICAR组,每组6只小鼠,连续干预5周后处死所有小鼠并收集血浆、肝脏组织等标本备用。
1.4 ELISA检测小鼠血浆肿瘤坏死因子α(tumor necrosis factor,TNF-α)和γ干扰素(interferon γ,IFN-γ)的浓度收集小鼠血浆,按ELISA检测试剂盒(美国eBioscience公司)说明书操作,检测血浆中TNF-α和IFN-γ的浓度。
1.5 肝功能和血脂测定收集小鼠血浆,用7170S全自动生化分析仪(日本Hitachi公司)检测丙氨酸转氨酶(alanine aminotransferase,ALT)、天冬氨酸转氨酶(aspartate aminotransferase,AST)、总胆固醇、三酰甘油和游离脂肪酸的浓度。
1.6 肝脏组织苏木精-伊红(H-E)染色和油红O染色用10%甲醛溶液固定肝脏组织后,石蜡包埋,切片,然后按H-E染色试剂盒(上海碧云天生物技术有限公司)和油红O染色试剂盒(美国ScienCell公司)说明书操作。
1.7 蛋白质印迹法检测AMPK蛋白表达用RIPA裂解液提取小鼠肝脏组织的总蛋白,BCA法测定总蛋白浓度,100 ℃蛋白变性10 min。SDS-聚丙烯酰胺凝胶电泳1.5 h,转膜1.5 h,封闭2 h,孵育AMPK一抗(美国Santa Cruz公司)过夜,漂洗8 min×3次,孵育二抗(美国Santa Cruz公司)1.5 h,漂洗8 min×3次后显影。采用Fluor-S成像系统(美国Bio-Rad公司)对条带进行扫描和定量分析,以GAPDH为内参照。
1.8 统计学处理采用SPSS 16.0软件进行数据处理。数据以x±s表示,两样本间均数的比较采用独立样本t检验,多个样本间比较采用单因素方差分析。检验水准(α)为0.05。
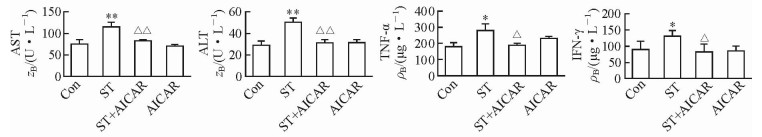
2 结果 2.1 小鼠肝功能和血浆促炎细胞因子浓度变化肝功能检测结果显示,ST组小鼠血浆AST和ALT浓度较Con组均升高[(114.45±13.99) U/L vs (76.91±4.55) U/L,(49.58±3.99) U/L vs (28.58±3.53) U/L;P均 < 0.01]。血浆促炎性细胞因子检测结果显示,ST组小鼠的血浆TNF-α和IFN-γ浓度较Con组均升高[(271.55±59.86) μg/L vs (174.55±16.28) μg/L,(131.1±18.84) μg/L vs (89.48±28.77) μg/L;P均 < 0.05]。而ST+AICAR组小鼠血浆AST浓度[(82.00±3.25) U/L]和ALT浓度[(30.80±3.12) U/L]较ST组均降低(P均 < 0.01),TNF-α[(174.37±4.70) μg/L]和IFN-γ[(82.09±25.64) μg/L]浓度较ST组也均降低(P均 < 0.05)。见图 1。
|
图 1 慢性应激对小鼠肝功能和血浆促炎细胞因子浓度的影响及AICAR的缓解作用 Fig 1 Effects of chronic stress on liver function and serum pro-inflammatory cytokine concentrations and the alleviating effects of AICAR Con: Control; ST: Stress; AICAR: 5-Aminoimidazole-4-carboxamide-ribonucleoside; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TNF-α: Tumor necrosis factor α; IFN γ: Interferon γ. *P < 0.05, **P < 0.01 vs Con group; △P < 0.05, △△P < 0.01 vs ST group. n=6, x±s |
2.2 小鼠血脂变化和肝细胞脂肪变性
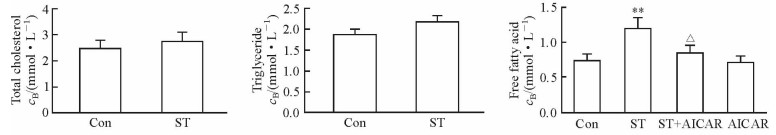
ST组小鼠的血浆游离脂肪酸浓度较Con组升高[(1.20±0.15) mmol/L vs (0.74±0.11) mmol/L,P < 0.01],总胆固醇和三酰甘油浓度与Con组相比差异均无统计学意义。ST小鼠给予AICAR处理后,血浆游离脂肪酸浓度较ST组降低[(0.84±0.25) mmol/L vs (1.20±0.15) mmol/L,P < 0.05]。见图 2。
|
图 2 慢性应激对小鼠血脂的影响及AICAR的缓解作用 Fig 2 Effects of chronic stress on serum lipids and the alleviating effects of AICAR Con: Control; ST: Stress; AICAR: 5-Aminoimidazole-4-carboxamide-ribonucleoside. **P < 0.01 vs Con group; △P < 0.05 vs ST group. n=6, x±s |
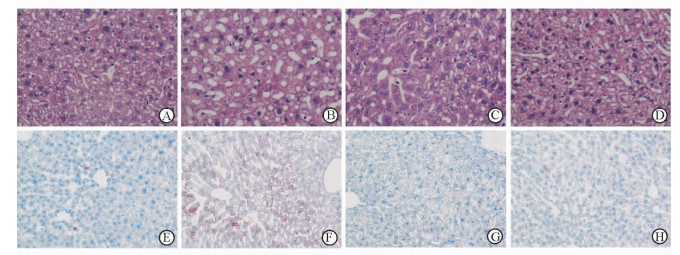
肝脏组织H-E染色(脂肪变性表现为空泡)和油红O染色(脂肪变性表现为红染)结果(图 3)显示,ST组小鼠肝细胞脂肪变性较Con组明显增加,而ST+AICAR组小鼠肝细胞脂肪变性较ST组明显减少。
|
图 3 慢性应激对小鼠肝细胞脂肪变性的影响及AICAR的缓解作用 Fig 3 Effect of chronic stress on hepatic stestosis and the alleviating effects of AICAR A, E: Control group; B, F: Stress group; C, G: Stress+AICAR group; D, H: AICAR group. A-D: H-E staining; E-H: Oil Red O staining. AICAR: 5-Aminoimidazole-4-carboxamide-ribonucleoside. Original magnification: ×400 |
2.3 小鼠肝组织AMPK蛋白表达变化
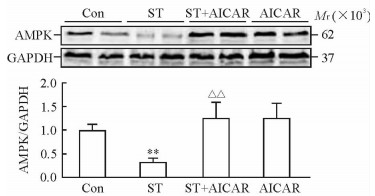
蛋白质印迹法检测结果显示,ST组小鼠的肝组织AMPK蛋白表达较Con组降低(P < 0.01),而ST+AICAR组小鼠肝组织AMPK蛋白表达较ST组升高(P < 0.01)。见图 4。
|
图 4 慢性应激对小鼠肝组织AMPK蛋白表达的影响及AICAR的阻断作用 Fig 4 Effect of chronic stress on AMPK expressions in liver and blocking effects of AICAR Con: Control; ST: Stress; AICAR: 5-Aminoimidazole-4-carboxamide-ribonucleoside; AMPK: Adenosine monophosphate-activated protein kinase; GAPDH: Glyceraldehydes-3-phosphate dehydrogenase. **P < 0.01 vs Con group; △△P < 0.01 vs ST group. n=6, x±s |
3 讨论
近年来,NAFLD的发病率逐年升高,已成为最常见的慢性肝病之一。NAFLD是以弥漫性肝细胞脂肪变性为主要特征的临床病理综合征,严重者可进展成为肝硬化和原发性肝细胞癌,严重威胁人们的健康[8]。
应激反应是机体应对外界环境刺激的重要生理反应,可激活下丘脑-垂体-肾上腺皮质(hypothalamus-pituitary-adrenal,HPA)轴,激动交感神经系统,引起广泛的神经-内分泌活动,调节机体代谢和免疫。慢性应激对机体健康的负面影响已被广泛报道,大量研究证实慢性应激能诱发和加剧各种慢性代谢性疾病和炎症疾病[9-11]。近年来有研究报道慢性应激可促进NAFLD的发生[3],但相关研究仍较少。目前对慢性应激引发NAFLD的作用及机制尚缺乏深入认识。
研究已证实炎性反应是NAFLD形成的重要原因[12-13],本实验发现慢性应激导致小鼠的血浆ALT、AST和促炎性细胞因子TNF-α、IFN-γ水平升高,提示慢性应激可诱发肝脏炎症和肝功能损害。AMPK和炎性反应密切相关[14],本实验发现慢性应激导致小鼠肝脏组织AMPK蛋白表达下降,给予AMPK激动剂AICAR可阻断慢性应激对肝脏AMPK蛋白表达的抑制。随着小鼠肝脏组织AMPK蛋白表达增加,血浆促炎性细胞因子水平降低,小鼠肝功能损害得到有效缓解,提示慢性应激可能通过AMPK信号通路上调血浆促炎性细胞因子的表达,从而促进NAFLD发生和发展。AMPK抑制炎性反应的机制可能是通过阻断NF-κB、MAPK和JAK-STAT等信号通路实现的[15-16]。
脂质代谢紊乱是NAFLD形成的另一个重要原因[3]。本实验发现慢性应激导致小鼠血浆游离脂肪酸浓度升高,肝脏细胞脂肪变性增加。AMPK是调节能量代谢的关键酶,对肝脏脂质代谢具有重要调节作用[4-5]。随着小鼠肝脏组织AMPK蛋白表达增加,血浆游离脂肪酸水平降低,小鼠肝细胞脂肪变性得到有效缓解,提示慢性应激可能通过AMPK信号通路调控血浆游离脂肪酸水平和肝脏细胞脂肪变性,从而促进NAFLD的发生和发展。AMPK调控肝脏脂代谢的可能机制包括通过降低Srebp1c和Fas活性抑制肝脏内胆固醇和三酰甘油的合成,以及通过减少乙酰辅酶A羧化酶活性促进脂肪酸的β氧化过程等[17-21]。
综上所述,本实验通过动物实验方法初步证实,慢性应激可能通过AMPK信号通路诱发NAFLD,其机制可能是慢性应激下调小鼠肝脏组织AMPK的表达,引起小鼠机体炎性反应和脂代谢障碍,进而导致小鼠肝功能损害和肝细胞脂肪变性,最终发生NAFLD。AMPK激动剂AICAR可有效阻断慢性应激对肝脏组织AMPK蛋白表达的抑制,从而缓解慢性应激导致的NAFLD。
| [1] | HACKETT R A, STEPTOE A. Psychosocial factors in diabetes and cardiovascular risk[J]. Curr Cardiol Rep, 2016, 18: 95–102. DOI: 10.1007/s11886-016-0771-4 |
| [2] | LIU Y Z, CHEN J K, ZHANG Y, WANG X, QU S, JIANG C L. Chronic stress induces steatohepatitis while decreases visceral fat mass in mice[J]. BMC Gastroenterol, 2014, 14: 106–111. DOI: 10.1186/1471-230X-14-106 |
| [3] | DAY E A, FORD R J, STEINBERG G R. AMPK as a therapeutic target for treating metabolic diseases[J]. Trends Endocrinol Metab, 2017, 28: 545–560. DOI: 10.1016/j.tem.2017.05.004 |
| [4] | SMITH B K, STEINBERG G R. AMP-activated protein kinase, fatty acid metabolism, and insulin sensitivity[J]. Curr Opin Clin Nutr Metab Care, 2017, 20: 248–253. DOI: 10.1097/MCO.0000000000000380 |
| [5] | TOWNSEND S A, NEWSOME P N. Review article:new treatments in non-alcoholic fatty liver disease[J]. Aliment Pharmacol Ther, 2017, 46: 494–507. DOI: 10.1111/apt.2017.46.issue-5 |
| [6] | SOZEN E, OZER N K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases:an updated mini-review[J]. Redox Biol, 2017, 12: 456–461. DOI: 10.1016/j.redox.2017.02.025 |
| [7] | PENG Y L, LIU Y N, LIU L, WANG X, JIANG C L, WANG Y X. Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress[J]. J Neuroinflammation, 2012, 9: 75–83. |
| [8] | DOYCHEVA I, WATT K D, ALKHOURI N. Nonalcoholic fatty liver disease in adolescents and young adults:the next frontier in the epidemic[J]. Hepatology, 2017, 65: 2100–2109. DOI: 10.1002/hep.29068 |
| [9] | AQEL S I, HAMPTON J M, BRUSS M, JONES K T, VALIENTE G R, WU L C, et al. Daily moderate exercise is beneficial and social stress is detrimental to disease pathology in murine lupus nephritis[J]. Front Physiol, 2017, 8: 236–243. DOI: 10.3389/fphys.2017.00236 |
| [10] | HALARIS A. Inflammation-associated co-morbidity between depression and cardiovascular disease[J]. Curr Top Behav Neurosci, 2017, 31: 45–70. |
| [11] | DHABHAR F S. Effects of stress on immune function:the good, the bad, and the beautiful[J]. Immunol Res, 2014, 58: 193–210. DOI: 10.1007/s12026-014-8517-0 |
| [12] | RALSTON J C, LYONS C L, KENNEDY E B, KIRWAN A M, ROCHE H M. Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues[J]. Annu Rev Nutr, 2017, 37: 77–102. DOI: 10.1146/annurev-nutr-071816-064836 |
| [13] | OSAYANDE A S, KALE N. Nonalcoholic fatty liver disease:identifying patients at risk of inflammation or fibrosis[J]. Am Fam Physician, 2017, 95: 796–797. |
| [14] | KIRWAN A M, LENIGHAN Y M, O'REILLY M E, MCGILLICUDDY F C, ROCHE H M. Nutritional modulation of metabolic inflammation[J]. Biochem Soc Trans, 2017, 45: 979–985. DOI: 10.1042/BST20160465 |
| [15] | RENA G, HARDIE D G, PEARSON E R. The mechanisms of action of metformin[J/OL]. Diabetologia, 2017 Aug 3. doi:10.1007/s00125-017-4342-z.[Epubaheadofprint] |
| [16] | UMEZAWA S, HIGURASHI T, NAKAJIMA A. AMPK:therapeutic target for diabetes and cancer prevention[J]. Curr Pharm Des, 2017, 23: 3629–3644. |
| [17] | IDETA T, SHIRAKAMI Y, MIYAZAKI T, KOCHI T, SAKAI H, MORIWAKI H, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice[J]. Int J Mol Sci, 2015, 16: 29207–29218. DOI: 10.3390/ijms161226156 |
| [18] | ALENAZI F S, IBRAHIM B A, AL-HAMAMI H, SHAKIYA M, BRISKI K P. Role of estradiol in intrinsic hindbrain AMPK regulation of hypothalamic AMPK, metabolic neuropeptide, and norepinephrine activity and food intake in the female rat[J]. Neuroscience, 2016, 314: 35–46. DOI: 10.1016/j.neuroscience.2015.11.048 |
| [19] | ZHOU X, HE L, ZUO S, ZHANG Y, WAN D, LONG C, et al. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes[J]. Biochim Biophys Acta, 2017, 1864: 488–498. |
| [20] | LEE S M, DOROTEA D, JUNG I, NAKABAYASHI T, MIYATA T, HA H. TM5441, a plasminogen activator inhibitor-1 inhibitor, protects against high fat diet-induced non-alcoholic fatty liver disease[J]. Oncotarget, 2017, 8: 89746–89760. |
| [21] | BOYLE K E, PATINKIN Z W, SHAPIRO A L B, BADER C, VANDERLINDEN L, KECHRIS K, et al. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants[J]. Mol Metab, 2017, 6: 1503–1516. DOI: 10.1016/j.molmet.2017.08.012 |
 2017, Vol. 38
2017, Vol. 38


