2. 重庆代谢性疾病转化医学重点实验室, 重庆 400016;
3. 重庆医科大学附属第一医院肾脏内科, 重庆 400016
2. Chongqing Key Laboratory of Translational Medicine in Major Metabolic Diseases, Chongqing 400016, China;
3. Department of Nephrology, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
动脉粥样硬化性心血管疾病是慢性肾脏病(chronic kidney disease,CKD)患者最常见的并发症和死亡原因之一[1-2]。高磷血症是CKD患者发生加速性动脉粥样硬化的独立危险因素,但其促进动脉粥样硬化的分子机制不清[3-4]。固醇调节元件结合蛋白2(sterol regulatory element binding protein 2,SREBP2)及其裂解激活蛋白(SREBP cleavage activating protein,SCAP)介导的负反馈调控系统对真核细胞内胆固醇稳态的调节具有关键作用[5],其功能异常与炎症、糖尿病等多种病理状态下外周细胞内脂质异常集聚有关[6-7]。本研究拟利用体外细胞培养模拟高磷血症,探讨高磷环境对巨噬细胞内源性胆固醇积聚的影响及其分子机制。
1 材料和方法 1.1 仪器与试剂人单核细胞株(human acute monocytic leukemia cell line,THP-1,No: TIB-202)购于美国ATCC公司,胎牛血清(fetal bovine serum,FBS)购于德国PAN公司,DMEM培养基、青/链霉素购于Hyclone公司,蛋白酶抑制剂、佛波酯(phorbol myristate acetate,PMA)、磷甲酸钠(phosphonoformic acid,PFA)、无脂肪酸牛血清白蛋白(bovine serum albumin,BSA)、磷酸二氢钠(NaH2PO4)、磷酸氢二钠(Na2HPO4)购于美国Sigma公司,培养瓶、培养皿购于NEST公司,PrimeScript RT反转录试剂盒购于TaKaRa公司,兔抗人SCAP多克隆一抗、兔抗人SREBP2多克隆一抗购于Abgent公司,兔抗人羟甲基戊二酸单酰辅酶A还原酶(3-hydroxy-3-methyl-glutaryl coenzyme A reductase,HMGCoAR)多克隆一抗购于美国Abcam公司,兔抗人低密度脂蛋白受体(low density lipoprotein receptor,LDLR)多克隆一抗购于武汉三鹰公司,兔抗小鼠、山羊抗兔辣根过氧化物酶标记的二抗购于北京中杉金桥生物技术有限公司,小鼠抗Golgin一抗、山羊抗兔绿色荧光二抗、山羊抗小鼠红色荧光二抗购于英国Molecular Probes公司。激光扫描共聚焦显微镜(LEICA TCS SP2)、正置高清显微镜(DM4000B)由德国Leica公司生产。其他试剂均为分析纯。
1.2 细胞培养及巨噬细胞处理THP-1细胞使用含10% FBS、100 U/mL青霉素/链霉素的DMEM培养液(生长培养培基),置于37 ℃、5% CO2孵箱中培养。均匀接种适量THP-1细胞于含160 nmol/L PMA的生长培养液中培养72 h,诱导分化其成为形态典型的M1型巨噬细胞[8]。使用含0.5% BSA和100 U/mL青霉素/链霉素的DMEM培养液作为实验培养液。将分化成熟的巨噬细胞用实验培养液预处理12 h后,使用pH为7.4的200 mmol/L NaH2PO4/Na2HPO4缓冲液配制不同磷浓度实验培养液,设置对照组(含磷1.0 mmol/L)、高磷处理组(含磷3.0 mmol/L)、PFA处理组(含磷1.0 mmol/L及PFA 1.0 mmol/L)、高磷联合PFA处理组(含磷3.0 mmol/L及PFA 1.0 mmol/L)处理细胞,置于37 ℃、5% CO2孵箱中培养24 h后待检。
1.3 巨噬细胞油红O染色将THP-1细胞接种于1 cm×1 cm玻片上,经诱导分化和不同处理24 h后收集各组细胞,4%多聚甲醛室温固定30 min,PBS清洗,1, 2-丙二醇孵育2 min后加入0.2%油红O染液染色15 min,苏木精染核,甘油明胶封片,正置显微镜高倍视野(×400)下观察拍照。
1.4 细胞内胆固醇定量测定将细胞接种于6孔板内,经诱导分化和处理后收集各组细胞悬液,离心去除上清,加入氯仿/甲醇溶液混匀,超声震碎后摇床室温震荡15 min,超高速离心,取上清液真空干燥后加入95%乙醇溶液,测量细胞内的游离胆固醇(free cholesterol,FC)及总胆固醇(total cholesterol,TC)含量,两者之差即为胆固醇酯(cholesterol ester,CE)含量。另取超高速离心沉淀,加入1.0 mol/L NaOH溶液至沉淀完全溶解,使用Lowry法测量蛋白浓度,以各组细胞蛋白含量标化胆固醇含量。
1.5 qPCR检测LDLR、HMGCoAR及SCAP mRNA的表达取THP-1细胞接种于6孔板中,诱导分化和给予不同处理后,TRIzol法提取各组细胞总RNA,用Nano 2000核酸蛋白测定仪测定RNA浓度并标准化。按照TaKaRa反转录试剂盒说明书将不同组等量RNA反转录为cDNA。以β-actin为内参,按照25 μL反应体系使用Opticon 2 real time PCR仪进行qPCR,检测SCAP、HMGCoAR、LDLR及β-actin mRNA的表达水平。引物序列如下:SCAP上游5′-GGG AAC TTC TGG CAG AAT GAC T-3′,下游5′-CTG GTG GAT GGT CCC AAT G-3′;LDLR上游5′-CTG TGG GCT CCA TAG GCT ATC T-3′,下游5′-GCG GTC CAG GGT CAT CTT C-3′;HMGCoAR上游5′-TCT GGC AGT CAG TGG GAA CTA TT-3′,下游5′-CCT CGT CCT TCG ATC CAA TTT-3′;β-actin上游5′-CCT GGC ACC CAG CAC AAT-3′,下游5′-GCC GAT CCA CAC GGA GTA CT-3′。qPCR反应条件为:50 ℃ 120 s,95 ℃ 300 s;95 ℃ 20 s,55 ℃ 20 s,共40次循环;95 ℃ 60 s,55 ℃ 60 s。以Ct值反应初始模板量,采用比较Ct值法(2-ΔΔCt)计算目的基因表达水平。
1.6 蛋白质印迹法检测LDLR、HMGCoAR、N-SREBP2及SCAP的蛋白水平各组巨噬细胞经不同处理后,PBS清洗3次,使用添加1%蛋白酶抑制剂的裂解液裂解细胞,提取总蛋白,按照核蛋白提取试剂盒说明书提取细胞核蛋白,使用Lowry法测定蛋白浓度并标准化。各组取等量蛋白(80~100 μg)上样,行聚丙烯酰胺凝胶电泳,250 mA 4 ℃转膜,5%脱脂奶粉室温封闭2 h,分别加入兔抗人HMGCoAR一抗(1:1 000)、兔抗人LDLR一抗(1:1 000)、兔抗人SCAP一抗(1:200)、兔抗人SREBP2一抗(1:1 000) 4 ℃孵育过夜,TBST洗涤3次,室温孵育辣根过氧化物酶标记的二抗(1:10 000)1 h,超敏ECL显影液及凝胶成像系统显影,以目标蛋白与β-actin条带的光密度值比值为其相对表达含量。
1.7 激光共聚焦法检测SCAP共定位取THP-1细胞接种于1 cm×1 cm玻片上,经诱导分化和不同处理后,4%多聚甲醛室温固定,0.25% Triton X-100透膜,10%山羊血清室温封闭30 min,加入兔抗人SCAP一抗(1:50)、小鼠抗Golgin一抗(1:50)室温孵育1 h,PBS清洗15~30 min,加入山羊抗兔绿色荧光二抗(1:100)、山羊抗小鼠红色荧光二抗(1:100)室温避光孵育60 min,PBST清洗3次后共聚焦显微镜下观察并拍摄照片。
1.8 统计学处理采用SPSS 18.0软件分析处理数据,结果以x±s表示,多个不同处理组定量指标比较采用单因素方差分析,组间两两比较采用两样本均数的t检验。检验水准(α)为0.05。
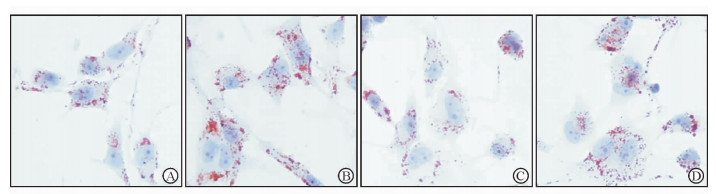
2 结果 2.1 THP-1源性巨噬细胞油红O染色高磷处理组巨噬细胞内中性脂质含量明显高于对照组,表现为红染颗粒更多、更大。PFA处理组巨噬细胞内中性脂质含量较对照组并无显著减少,但高磷环境下PFA处理(高磷联合PFA处理组)明显抑制了高磷引起的细胞内脂质蓄积。见图 1。
|
图 1 油红O染色观察高磷及PFA对巨噬细胞内脂质蓄积的影响 Fig 1 Effects of high phosphate condition and PFA on neutral lipid accumulation in macrophage by Oil Red O staining A: Normal control group, phosphate 1.0 mmol/L; B: High phosphate group, phosphate 3.0 mmol/L; C: PFA treatment group, phosphate 1.0 mmol/L+PFA 1.0 mmol/L; D: High phosphate plus PFA treatment group, phosphate 3.0 mmol/L+PFA 1.0 mmol/L. PFA: Phosphonoformic acid. Original magnification: ×400 |
2.2 细胞内胆固醇定量测定
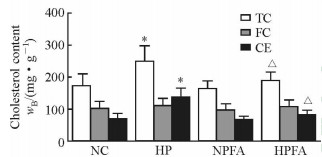
使用酶催化比色法定量测定细胞内胆固醇含量,消除油红O染色法中脂肪酸造成的假阳性。结果(图 2)显示,高磷处理组巨噬细胞内TC和CE含量较对照组均增加(P < 0.05),而FC含量在两组间差异无统计学意义(P>0.05)。与单纯高磷处理组相比,高磷联合PFA处理组巨噬细胞内TC和CE含量降低(P < 0.05),接近对照组的水平。
|
图 2 高磷及PFA对巨噬细胞内胆固醇含量的影响 Fig 2 Effects of high phosphate condition and PFA on cholesterol contents in macrophage NC: Normal control group, phosphate 1.0 mmol/L; HP: High phosphate group, phosphate 3.0 mmol/L; NPFA: PFA treatment group, phosphate 1.0 mmol/L+PFA 1.0 mmol/L; HPFA: High phosphate plus PFA treatment group, phosphate 3.0 mmol/L+PFA 1.0 mmol/L. PFA: Phosphonoformic acid; TC: Total cholesterol; FC: Free cholesterol; CE: Cholesterol ester. *P < 0.05 vs NC group; △P < 0.05 vs HP group. n=3, x±s |
2.3 高磷状态下巨噬细胞内LDLR、HMGCoAR mRNA水平增高
高磷处理组巨噬细胞内HMGCoAR、LDLR mRNA的表达水平较对照组均升高(P < 0.01);而单独PFA处理对HMGCoAR、LDLR mRNA的表达水平无明显影响。与高磷处理组相比,高磷环境下PFA处理能抑制高磷导致的HMGCoAR、LDLR mRNA表达上调(P < 0.01,图 3)。

|
图 3 高磷与PFA对巨噬细胞LDLR与HMGCoAR mRNA表达水平的影响 Fig 3 Effects of high phosphate condition and PFA on the LDLR and HMGCoAR mRNA expressions in macrophage NC: Normal control group, phosphate 1.0 mmol/L; HP: High phosphate group, phosphate 3.0 mmol/L; NPFA: PFA treatment group, phosphate 1.0 mmol/L+PFA 1.0 mmol/L; HPFA: High phosphate plus PFA treatment group, phosphate 3.0 mmol/L+PFA 1.0 mmol/L. PFA: Phosphonoformic acid; LDLR: Low density lipoprotein receptor; HMGCoAR: 3-Hydroxy-3-methyl-glutaryl coenzyme A reductase. **P < 0.01 vs NC group; △△P < 0.01 vs HP group. n=3, x±s |
2.4 高磷状态下巨噬细胞内LDLR、HMGCoAR及核内N-SREBP2蛋白水平增高
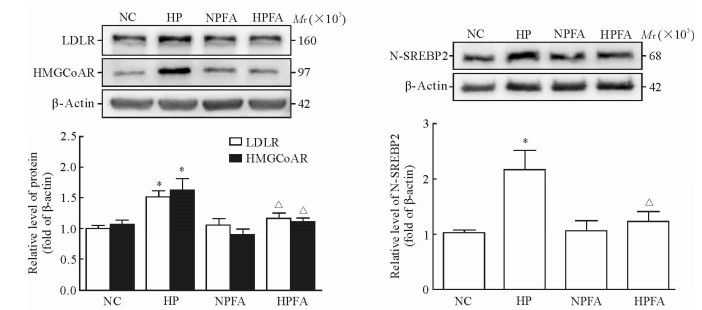
蛋白质印迹法检测结果显示,高磷处理组巨噬细胞内LDLR、HMGCoAR的蛋白表达水平较对照组增加(P < 0.05),而单独PFA处理对巨噬细胞LDLR、HMGCR蛋白的表达水平无明显影响(P>0.05);高磷下PFA处理(高磷联合PFA处理组)可抵消高磷导致的上述作用(P < 0.05)。进一步检测细胞核内SREBP2活化片段N-SREBP2的蛋白含量,发现高磷处理增加了N-SREBP2的蛋白水平(P < 0.05),单独PFA处理对N-SREBP2的蛋白水平无明显影响,而高磷下PFA处理(高磷联合PFA处理组)可抵消高磷导致的上述作用(P < 0.05)。见图 4。
|
图 4 高磷与PFA对巨噬细胞LDLR、HMGCoAR及核内N-SREBP2蛋白水平的影响 Fig 4 Effects of high phosphate condition and PFA on the protein level of LDLR、HMGCoAR and nuclear N-SREBP2 in macrophage NC: Normal control group, phosphate 1.0 mmol/L; HP: High phosphate group, phosphate 3.0 mmol/L; NPFA: PFA treatment group, phosphate 1.0 mmol/L+PFA 1.0 mmol/L; HPFA: High phosphate plus PFA treatment group, phosphate 3.0 mmol/L+PFA 1.0 mmol/L. PFA: Phosphonoformic acid; LDLR: Low-density lipoprotein receptor; HMGCoAR: 3-Hydroxy-3-methyl-glutaryl coenzyme A reductase; N-SREBP2: Nuclear sterol regulatory element binding protein 2. *P < 0.05 vs NC group; △P < 0.05 vs HP group. n=3, x±s |
2.5 高磷状态下巨噬细胞内SCAP向高尔基体的移位增加
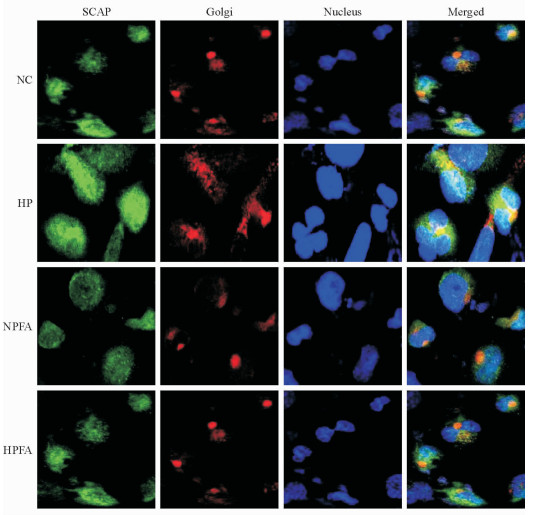
激光共聚焦检测结果(图 5)发现,高磷条件下巨噬细胞内SCAP的荧光强度增加,且SCAP与高尔基体共定位增多;加入PFA处理后,上述变化消失。单独PFA处理对巨噬细胞内SCAP荧光强度及其与高尔基体共定位的影响均不大。
|
图 5 高磷及PFA对巨噬细胞内SCAP与高尔基体共定位的影响 Fig 5 Effects of high phosphate condition and PFA on protein translocation of SCAP from endoplasmic reticulum to Golgi in macrophage NC: Normal control group, phosphate 1.0 mmol/L; HP: High phosphate group, phosphate 3.0 mmol/L; NPFA: PFA treatment group, phosphate 1.0 mmol/L+PFA 1.0 mmol/L; HPFA: High phosphate plus PFA treatment group, phosphate 3.0 mmol/L+PFA 1.0 mmol/L. PFA: Phosphonoformic acid; SCAP: Sterol regulatory element binding protein cleavage activating protein. Confocal microscopy. Original magnification: ×400 |
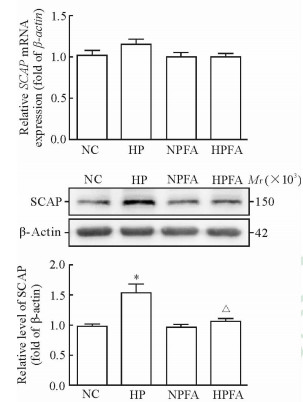
2.6 高磷处理增高巨噬细胞内SCAP的蛋白水平
与对照组相比,高磷处理组巨噬细胞内SCAP蛋白水平增高(P < 0.05);PFA处理能减弱高磷的上述作用(P < 0.05),但对正常磷环境下SCAP的蛋白水平无影响(P>0.05)。SCAP mRNA的表达水平在各处理组间差异均无统计学意义。见图 6。
|
图 6 高磷与PFA对巨噬细胞SCAP mRNA及蛋白水平的影响 Fig 6 Effects of high phosphate condition and PFA on the mRNA and protein levels of SCAP in macrophage NC: Normal control group, phosphate 1.0 mmol/L; HP: High phosphate group, phosphate 3.0 mmol/L; NPFA: PFA treatment group, phosphate 1.0 mmol/L+PFA 1.0 mmol/L; HPFA: High phosphate plus PFA treatment group, phosphate 3.0 mmol/L+PFA 1.0 mmol/L. PFA: Phosphonoformic acid; SCAP: Sterol regulatory element binding protein cleavage activating protein. *P < 0.05 vs NC group; △P < 0.05 vs HP group. n=3, x±s |
3 讨论
CKD患者的动脉粥样硬化病变发生早、进展快,又被称为“加速性动脉粥样硬化”,其引起的心血管疾病是CKD尤其是终末期肾脏病患者最主要的并发症和死亡原因之一[1, 9],阐明CKD患者动脉粥样硬化的分子机制对探索新的防治策略具有重要意义。人体正常血磷范围为0.8~1.5 mmol/L,CKD患者由于磷排泄功能异常,磷代谢明显紊乱,高磷血症的发生率高达70%~80%[2]。近年来,众多研究表明血磷水平增高是动脉粥样硬化的重要独立危险因素[4, 10-11],但其分子机制目前仍不清楚。
胆固醇在血管壁单核源性巨噬细胞和血管平滑肌细胞内大量异常积聚,促使其形成泡沫细胞是动脉粥样斑块形成的基本病理过程[12]。越来越多的临床研究提示,血磷可能具有与胆固醇相似的致动脉粥样硬化作用[10, 13]。本研究利用THP-1源性巨噬细胞探讨高磷环境对泡沫细胞形成的影响及分子机制,THP-1细胞可经PMA诱导分化为巨噬细胞,能较快蓄积大量脂质,在动脉粥样硬化泡沫细胞形成及脂质代谢紊乱的研究中广泛应用[7-8]。鉴于高磷血症患者的血磷浓度多为1.5~3.0 mmol/L[14],而PFA可有效阻断磷离子通过细胞表面的钠磷转运体Pit-1进入细胞内[15],本研究设置高磷处理(含磷3.0 mmol/L)及高磷联合PFA等干预巨噬细胞,结果发现高磷环境下巨噬细胞内CE明显积聚,而PFA能有效抑制上述变化且对正常磷环境中的巨噬细胞无明显影响。结果表明高磷血症可能是通过增加细胞内磷离子浓度干扰细胞内胆固醇的稳态平衡,促进THP-1源性巨噬细胞泡沫化。
生理状态下真核细胞内的胆固醇稳态平衡受SCAP-SREBP2介导的负反馈调控系统的控制[5]。SCAP是一种内质网膜蛋白,其氨基端含有一个固醇敏感区,能感受细胞内胆固醇水平的变化,因此SCAP也被认为是一种细胞内的胆固醇敏感器[5, 16]。在细胞内,SCAP可与SREBP2紧密结合形成复合物。当细胞内胆固醇缺乏时,SCAP的固醇敏感区感受到这一变化,随即发生空间构象改变,作为活性伴侣分子运载SREBP2至高尔基体进行酶解,释放其氨基端活性片段N-SREBP2,后者转位入核并与HMGCoAR和LDLR基因启动子区的固醇调节元件(sterol regulatory element, SRE)结合,促进HMGCoAR与LDLR基因转录,上调HMGCoAR与LDLR的蛋白水平,最终导致细胞内胆固醇水平增加。反之,当细胞内胆固醇充足时,SCAP处于非活化空间构象,与SREBP2形成复合物而将其滞留在内质网上,从而抑制HMGCoAR与LDLR的蛋白表达,不会导致细胞内胆固醇水平的增加[17]。在炎症、糖尿病等病理条件下,上述调节机制“失灵”是肝细胞[18]、血管平滑肌细胞[8]等多种外周细胞内胆固醇异常积聚的重要病理机制。
本研究采用qPCR和蛋白质印迹法检测发现,高磷处理后THP-1源性巨噬细胞内HMGCoAR和LDLR mRNA和蛋白的表达水平均增高,核内N-SREBP2的含量也增加,而PFA能有效抵消高磷引起的上述变化。结果表明,高磷环境下THP-1源性巨噬细胞对HMGCoAR和LDLR表达的负反馈调控机制可能处于“失灵”状态。研究进一步检测了SCAP与高尔基体的共定位以及SCAP mRNA与蛋白的表达水平,结果显示高磷环境下SCAP的蛋白水平升高,且其与高尔基体共定位显著增加,而PFA处理可抵消这一变化,同时高磷或PFA单独处理均不影响SCAP mRNA的表达,表明细胞内磷浓度增高后,可能通过某种转录后调节机制增加了SCAP的蛋白水平,导致SCAP-SREBP2由内质网向高尔基体移位增加。目前,异常糖基化修饰是唯一已知的可在转录后水平异常调节SCAP功能的机制[7],且被证实与肿瘤发生、HMC细胞内脂质积聚有关[19],但限于糖蛋白富集过程中的糖链丢失、糖蛋白质谱分析技术难度高等条件的制约,目前SCAP蛋白异常糖基化修饰与其功能失调的相关研究还处于初步探索阶段。
综上所述,高磷环境下磷通过细胞表面Pit-1进入细胞,在转录后阶段增加SCAP蛋白水平并诱导其功能失调,从而异常运载SREBP2至高尔基体裂解生成过多N-SREBP2,后者转位入核上调HMGCoAR及LDLR的表达,促进巨噬细胞内源性胆固醇的合成和外源性LDL经LDLR摄取细胞内,最终导致巨噬细胞内胆固醇大量积聚及泡沫化。这可能是CKD合并高磷血症患者加速性动脉粥样硬化的发病机制之一。
| [1] | ENE-IORDACHE B, PERICO N, BIKBOV B, CARMINATI S, REMUZZI A, PERNA A, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC):a cross-sectional study[J/OL]. Lancet Glob Health, 2016, 4:e307-e319. doi:10.1016/s2214-109x(16)00071-1. |
| [2] | LEVIN A, STEVENS P E. Summary of KDIGO 2012 CKD Guideline:behind the scenes, need for guidance, and a framework for moving forward[J]. Kidney Int, 2014, 85: 49–61. DOI: 10.1038/ki.2013.444 |
| [3] | CHANG A R, LAZO M, APPEL L J, GUTIERREZ O M, GRAMS M E. High dietary phosphorus intake is associated with all-cause mortality:results from NHANES Ⅲ[J]. Am J Clin Nutr, 2014, 99: 320–327. DOI: 10.3945/ajcn.113.073148 |
| [4] | PARK K S, PARK J, CHOI S H, ANN S H, SINGH G B, SHIN E S, et al. Serum phosphorus concentration and coronary artery calcification in subjects without renal dysfunction[J/OL]. PLoS One, 2016, 11:e0151007. doi:10.1371/journal.pone.0151007. |
| [5] | GOLDSTEIN J L, DEBOSE-BOYD R A, BROWN M S. Protein sensors for membrane sterols[J]. Cell, 2006, 124: 35–46. DOI: 10.1016/j.cell.2005.12.022 |
| [6] | MA K L, LIU J, WANG C X, NI J, ZHANG Y, WU Y, et al. Activation of mTOR modulates SREBP-2 to induce foam cell formation through increased retinoblastoma protein phosphorylation[J]. Cardiovasc Res, 2013, 100: 450–460. DOI: 10.1093/cvr/cvt203 |
| [7] | ZHOU C, LEI H, CHEN Y, LIU Q, LI L C, MOORHEAD J F, et al. Enhanced SCAP glycosylation by inflammation induces macrophage foam cell formation[J/OL]. PLoS One, 2013, 8:e75650. doi:10.1371/journal.pone.0075650. |
| [8] | YE Q, LEI H, FAN Z, ZHENG W, ZHENG S. Difference in LDL receptor feedback regulation in macrophages and vascular smooth muscle cells:foam cell transformation under inflammatory stress[J]. Inflammation, 2014, 37: 555–565. DOI: 10.1007/s10753-013-9769-x |
| [9] | TANAKA K, WATANABE T, TAKEUCHI A, OHASHI Y, NITTA K, AKIZAWA T, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease[J]. Kidney Int, 2017, 91: 227–234. DOI: 10.1016/j.kint.2016.09.015 |
| [10] | ELLAM T J, CHICO T J. Phosphate:the new cholesterol? The role of the phosphate axis in non-uremic vascular disease[J]. Atherosclerosis, 2012, 220: 310–318. DOI: 10.1016/j.atherosclerosis.2011.09.002 |
| [11] | TONELLI M, SACKS F, PFEFFER M, GAO Z, CURHAN G, CHOLESTEROL, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease[J]. Circulation, 2005, 112: 2627–2633. DOI: 10.1161/CIRCULATIONAHA.105.553198 |
| [12] | SORCI-THOMAS M G, THOMAS M J. Microdomains, inflammation, and atherosclerosis[J]. Circ Res, 2016, 118: 679–691. DOI: 10.1161/CIRCRESAHA.115.306246 |
| [13] | YAMADA S, TOKUMOTO M, TATSUMOTO N, TANIGUCHI M, NOGUCHI H, NAKANO T, et al. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia[J]. Am J Physiol Renal Physiol, 2014, 306: 1418–1428. DOI: 10.1152/ajprenal.00633.2013 |
| [14] | BLOCK G A, KLASSEN P S, LAZARUS J M, OFSTHUN N, LOWRIE E G, CHERTOW G M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis[J]. J Am Soc Nephrol, 2004, 15: 2208–2218. DOI: 10.1097/01.ASN.0000133041.27682.A2 |
| [15] | LI X, YANG H Y, GIACHELLI C M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification[J]. Circ Res, 2006, 98: 905–912. DOI: 10.1161/01.RES.0000216409.20863.e7 |
| [16] | YE J, DEBOSE-BOYD R A. Regulation of cholesterol and fatty acid synthesis[J]. Cold Spring Harb Perspect Biol, 2011, 3: 1–13. DOI: 10.1101/cshperspect.a004754 |
| [17] | SATO R. Sterol metabolism and SREBP activation[J]. Arch Biochem Biophys, 2010, 501: 177–181. DOI: 10.1016/j.abb.2010.06.004 |
| [18] | LIU J, MA K L, ZHANG Y, WU Y, HU Z B, LV L L, et al. Activation of mTORC1 disrupted LDL receptor pathway:a potential new mechanism for the progression of non-alcoholic fatty liver disease[J]. Int J Biochem Cell Biol, 2015, 61: 8–19. DOI: 10.1016/j.biocel.2015.01.011 |
| [19] | CHENG C, RU P, GENG F, LIU J, YOO J Y, WU X, et al. Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth[J]. Cancer Cell, 2015, 28: 569–581. DOI: 10.1016/j.ccell.2015.09.021 |
 2017, Vol. 38
2017, Vol. 38


