2. 中国科学院深圳先进技术研究院, 深圳 518055
2. Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, Guangdong, China
高强度聚焦超声(high-intensity focused ultrasound, HIFU)在临床上广泛用于治疗肿瘤[1-2]。近年有关提高HIFU治疗效果的研究多集中在增强HIFU对肿瘤的定位成像和增强HIFU对肿瘤的杀伤力两个方面。当前临床上定位成像主要通过超声(ultrasound)或磁共振成像(magnetic resonance imaging,MRI)实现[3-6],评估疗效主要通过超声造影(contrast-enhanced ultrasound, CEUS)或MRI造影实现[7-9],增强疗效主要通过静脉注射超声造影剂或脂质体载纳米材料实现[10-12]。MRI对病灶整体及其与周边组织关系的显示较超声更有优势。然而,目前临床使用的MRI造影剂一般通过静脉注射,在肿瘤内的存留时间较短,不利于HIFU定位治疗;此外,定位、增强疗效及评估疗效均为独立程序,步骤繁琐、操作复杂。因此,研制一种集提高可视化精度定位、增强疗效及评估疗效于一体的纳米材料造影剂,对HIFU的临床意义重大。
介孔二氧化硅纳米囊脂质体(mesoporous silica nanocapsules loaded liposomes,MSN-LIPO)是一种纳米颗粒,可以通过被动靶向效应在肿瘤部位持续蓄积达24 h,便于完成HIFU治疗的整个过程,其所包封的乙酸异戊酯在HIFU作用下由液态转为气态,从而对HIFU起到显著的增强疗效作用。本科室前期构建了一种同时包封乙酸异戊酯和Fe3O4的MSN-LIPO,揭示了其基本理化特征、生物相容性及被人恶性胶质母细胞瘤细胞株U-87 MG的摄取情况,证实了其对HIFU灭活肿瘤细胞的增强作用[13]。但MSN-LIPO的MRI弛豫特征尚待验证,本研究旨在探索MSN-LIPO增强MRI显影的特点。
1 材料和方法 1.1 仪器与试剂核磁共振成像系统(Magnetom Trio,A Tim System 3T MRI System,德国西门子公司);自制MSN-LIPO[13];PBS(美国Hyclone公司);琼脂粉(天津市大茂化学试剂厂);人恶性胶质母细胞瘤细胞株U-87 MG细胞(ATCC® HTB-14TM,美国ATCC细胞库)。
1.2 体外MRI的弛豫特性检测将含铁浓度分别为0.16、0.08、0.04、0.02、0.01、0.005、0.002 5、0.001 25 mmol/L的MSN-LIPO溶液和纯水分别装入到1.5 mL密封离心管中,按照浓度从高到低的顺序依次放入含凝固琼脂的扫描盒孔中,进行MRI扫描。扫描参数:4通道小鼠线圈,自旋回波(spin echo,SE)序列,重复时间(repetition time,TR) 4 000 ms,回波时间(echo time,TE) 17.4 ms,翻转角(flip angle,Flip) 180°,层厚5 mm,视野(field of vision,FOV) 90 mm×180 mm。检测信号强度(signal intensity,SI)及T2值,以铁浓度为横坐标、1/T2为纵坐标绘制弛豫率曲线。
1.3 荷瘤裸鼠模型的构建SPF级BALB/c裸鼠6只,雌性,6~8周龄,体质量18~22 g,购于广东省医学实验动物中心[动物生产许可证:SCXK(粤)2013-0002,动物使用许可证:SYXK(粤)2012-0119]。饲料、饮水、垫料均经高压蒸汽灭菌处理(121 ℃、20 min),自由饮食。采用含10%胎牛血清、1%青霉素/链霉素的DMEM高糖培养液、于37 ℃、5% CO2的细胞培养箱中培养U-87 MG细胞。取对数生长期的U-87 MG细胞,胰酶消化1~2 min,1 000 r/min(r=15 cm)离心4 min,弃上清液后加入PBS重悬,按照1×107/只裸鼠的细胞密度接种于裸鼠侧腹部,隔天观察肿瘤生长情况。
1.4 荷瘤裸鼠MRI扫描 1.4.1 瘤内注射取3只荷瘤裸鼠麻醉固定,沿着肿瘤0、3、6、9点方向进针,注射200 μL含铁浓度为0.16 mmol/L的MSN-LIPO溶液,轻柔按压。瘤内注射前、注射后5 min内行MRI扫描。扫描参数:4通道小鼠线圈,SE序列,TR 3 700 ms,TE 71 ms,Flip 120°,层厚1.2 mm,FOV 48 mm×80 mm。
1.4.2 静脉注射另取3只荷瘤裸鼠麻醉固定,经尾静脉注射200 μL含铁浓度为0.16 mmol/L的MSN-LIPO溶液。静脉注射前、注射后30 min行MRI扫描。扫描参数同1.4.1项。
1.5 统计学处理采用SPSS 21.0软件进行数据分析。呈正态分布的计量资料以x±s表示,两组间比较采用独立样本t检验。检验水准(α)为0.05。
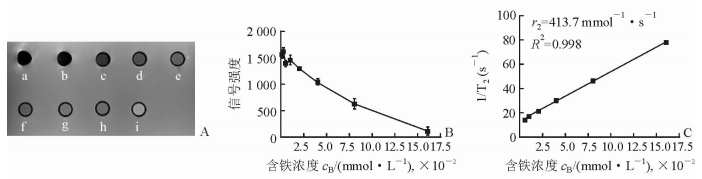
2 结果 2.1 MSN-LIPO体外MRI T2加权成像的弛豫特性不同含铁浓度MSN-LIPO的MRI扫描结果(图 1A)和定量分析结果(图 1B)显示,随着MSN-LIPO含铁浓度的逐渐增加,MRI T2信号强度呈逐渐降低的趋势。弛豫率曲线(图 1C)显示,1/T2在一定的MSN-LIPO含铁浓度范围内与铁浓度的呈线性关系,测得弛豫率(r2)为413.7 mmol-1·s-1。
|
图 1 MSN-LIPO体外MRI T2加权成像弛豫特性 A: MRI T2加权成像(a~h的含铁浓度分别为0.16、0.08、0.04、0.02、0.01、0.005、0.002 5、0.001 25 mmol/L,i为纯水);B:MSN-LIPO体外MRI模型扫描定量图(n=3, x±s);C:不同含铁浓度MSN-LIPO的弛豫效能拟合曲线. MRI:磁共振成像;MSN-LIPO:介孔二氧化硅纳米囊脂质体 |
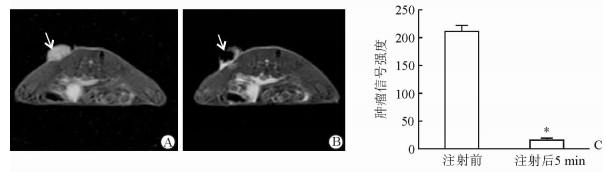
2.2 瘤内注射MSN-LIPO负性增强BALB/c荷瘤裸鼠皮下瘤MRI T2信号
注射前BALB/c荷瘤裸鼠肿瘤部位呈现T2高信号,肿瘤局部注射MSN-LIPO 5 min后,注射中心区域的信号强度降低,与周围组织呈现明显对比(图 2A、2B)。注射前肿瘤信号强度平均值为211.44±5.34,高于注射后5 min的15.34±1.24,差异有统计学意义(t=36.38,P < 0.05;图 2C)。
|
图 2 瘤内注射MSN-LIPO增强恶性胶质母细胞瘤MRI T2加权像及信号值 A:注射前,肿瘤呈高信号(箭头所示);B:注射后5 min肿瘤信号降低(箭头所示);C:注射MSN-LIPO前后肿瘤的MRI信号强度值. MRI:磁共振成像;MSN-LIPO:介孔二氧化硅纳米囊脂质体. *P < 0.05与注射前比较.n=3, x±s |
2.3 静脉注射MSN-LIPO负性增强BALB/c荷瘤裸鼠皮下瘤MRI T2信号
注射前BALB/c荷瘤裸鼠肿瘤部位呈现T2高信号,静脉注射MSN-LIPO 30 min后整个肿瘤区域的信号均降低,与注射前呈现明显对比(图 3A、3B)。注射前肿瘤信号强度平均值为235.99±5.17,高于注射后30 min的179.00±4.35,差异有统计学意义(t=14.34,P < 0.05;图 3C)。

|
图 3 静脉注射MSN-LIPO增强恶性胶质母细胞瘤瘤的MRI T2加权像及信号值 A:注射前,肿瘤呈高信号(箭头所示);B:注射后30 min肿瘤信号降低(箭头所示);C:注射MSN-LIPO前后肿瘤的MRI信号强度值. MRI:磁共振成像;MSN-LIPO:介孔二氧化硅纳米囊脂质体. *P < 0.05与注射前比较.n=3, x±s |
3 讨论
本研究所构建的MSN-LIPO是以乙酸异戊酯为HIFU增敏剂内核、Fe3O4为MRI造影剂的脂质体包封的纳米颗粒。乙酸异戊酯熔点为-78 ℃,沸点为143 ℃,常温为液态,在HIFU的作用下持续发生液-气相转换,形成微气泡,增强HIFU消融效应,从而增强HIFU对肿瘤的杀伤力。目前HIFU治疗的引导技术为超声或MRI。虽然MRI较超声检查时间长、费用高,但图像整体性好,能更清晰地分辨肿瘤与周围正常组织的界限,更精确地引导HIFU对病灶进行热消融,准确评估疗效。含Fe3O4的MRI造影剂具有超顺磁性、性质稳定、生物相容性好、强度高、无毒副作用等特点,被广泛应用于临床[14-15]。本科室前期研究证实MSN-LIPO具有对HIFU的增效作用[13],但在MRI T2加权成像中的效果尚不确定。本研究旨在验证MSN-LIPO的MRI T2加权成像增强效果。
本研究体外实验结果显示,MSN-LIPO具有良好的MRI T2加权成像增强功能。弛豫时间的长短决定着信号的强弱。MRI T2信号随着MSN-LIPO含铁浓度的降低呈增加趋势,1/T2与铁浓度呈现线性关系。根据MSN-LIPO的弛豫率曲线,测得弛豫率为413.7 mmol-1·s-1。可见MSN-LIPO因含Fe3O4而具有MRI造影剂功能,对临床上HIFU的精确引导具有潜在的应用价值。
本研究瘤内注射结果表明,MRI T2加权图像可清晰显示肿瘤内部MSN-LIPO的分布情况。对于浅表部位的肿瘤,局部直接注射可使瘤内药物保持高浓度状态,从而提高疗效。但瘤内注射也存在分布不均的现象,为了解浅表肿瘤内MSN-LIPO的分布情况,MRI T2加权成像非常必要。本研究中在瘤内局部注射MSN-LIPO后,肿瘤各部位T2信号强度相差悬殊,中心区域信号值极低,周围则表现为强信号,两者在T2加权图像上形成鲜明对比。这可能是由于肿瘤中心部位有较高浓度的MSN-LIPO而在T2加权图像上呈低信号,而周围部位MSN-LIPO扩散较少或无扩散而呈强信号,这不仅为HIFU提供了良好的消融定位,还可指导医师及时补充注射、补充消融。
本研究静脉注射结果表明,MSN-LIPO可增加MRI T2加权图像的肿瘤显影效果。对于深层及全身转移的肿瘤,不便于穿刺直接注射,经静脉注射药物是常用方法。目前临床使用的顺磁性静脉内造影剂钆喷酸葡胺(Gd-DTPA)或超顺磁性氧化铁(SPIO)不具备被动靶向功能,在肿瘤内停留时间短。而HIFU定位治疗用时较长,故Gd-DTPA与SPIO均不能用于引导HIFU消融治疗。MSN-LIPO是一种纳米颗粒,可通过被动靶向作用在肿瘤部位较长时间聚集[13],便于引导HIFU完成整个消融过程。尾静脉注射MSN-LIPO后,肿瘤部位的MRI T2信号值也会发生改变,显示在图像上的差异可被肉眼分辨,因此,对于需要静脉注射的肿瘤疾病,MSN-LIPO可以发挥良好的MRI T2加权像造影剂功能。然而,静脉注射MSN-LIPO引起的肿瘤组织T2信号降低水平明显不如瘤内注射,这可能是因为MSN-LIPO经静脉注射进入体内,除了被稀释外,肝、脾等脏器的主动摄取也会导致其在肿瘤部位的蓄积量减少。
综上所述,本研究从体外、瘤内注射和静脉注射3个层面验证了MSN-LIPO具有良好的MRI T2加权成像的造影功能,表明MSN-LIPO可以作为HIFU消融的造影剂。
| [1] | SCHULMAN A A, TAY K J, ROBERTSON C N, POLASCIK T J. High-intensity focused ultrasound for focal therapy:reality or pitfall?[J]. Curr Opin Urol, 2017, 27: 138–148. DOI: 10.1097/MOU.0000000000000372 |
| [2] | MARINOVA M, RAUCH M, SCHILD H H, STRUNK H M. Novel non-invasive treatment with high-intensity focused ultrasound (HIFU)[J]. Ultraschall Med, 2016, 37: 46–55. |
| [3] | LUO J, REN X, YU T. Efficacy of extracorporeal ultrasound-guided high intensity focused ultrasound:an evaluation based on controlled trials in China[J]. Int J Radiat Biol, 2015, 91: 480–485. |
| [4] | VIDAL-JOVE J, PERICH E, DEL CASTILLO M A. Ultrasound Guided High Intensity Focused Ultrasound for malignant tumors:the Spanish experience of survival advantage in stage Ⅲ and Ⅳ pancreatic cancer[J]. Ultrason Sonochem, 2015, 27(Suppl 1): 703–706. |
| [5] | EBBINI E S, TER HAAR G. Ultrasound-guided therapeutic focused ultrasound:current status and future directions[J]. Int J Hyperthermia, 2015, 31(Suppl 1): 77–89. |
| [6] | DE SENNEVILLE B D, MOONEN C, RIES M. MRI-guided HIFU methods for the ablation of liver and renal cancers[J]. Adv Exp Med Biol, 2016, 880: 43–63. DOI: 10.1007/978-3-319-22536-4 |
| [7] | CHENG C Q, ZHANG R T, XIONG Y, CHEN L, WANG J, HUANG G H, et al. Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases:retrospective analysis of contrast safety[J/OL]. Medicine (Baltimore), 2015, 94:e729. doi:10.1097/MD.0000000000000729. |
| [8] | PENG S, HU L, CHEN W, CHEN J, YANG C, WANG X, et al. Intraprocedure contrast enhanced ultrasound:the value in assessing the effect of ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids[J]. Ultrasonics, 2015, 58: 123–128. DOI: 10.1016/j.ultras.2015.01.005 |
| [9] | ZHAO W P, CHEN J Y, CHEN W Z. Dynamic contrast-enhanced MRI serves as a predictor of HIFU treatment outcome for uterine fibroids with hyperintensity in T2-weighted images[J]. Exp Ther Med, 2016, 11: 328–334. DOI: 10.3892/etm.2015.2879 |
| [10] | 陈莉, 胡兵. HIFU联合超声微泡造影剂治疗肿瘤的研究进展[J]. 中国医学影像学杂志, 2011, 19: 158–160. DOI: 10.3969/j.issn.1005-5185.2011.02.022 |
| [11] | WANG X, CHEN H, CHEN Y, MA M, ZHANG K, LI F, et al. Perfluorohexane-encapsulated mesoporous silica nanocapsules as enhancement agents for highly efficient high intensity focused ultrasound (HIFU)[J]. Adv Mater, 2012, 24: 785–791. DOI: 10.1002/adma.201104033 |
| [12] | LI J, KRUPKA T, YAO J, WANG R, JIANG L, ZHOU Y, et al. Liquid-solid phase-inversion PLGA implant for the treatment of residual tumor tissue after HIFU ablation[J/OL]. PLoS One, 2015, 10:e117358. doi:10.1371/journal.pone.0117358. |
| [13] | 唐家伟, 严飞, 段琬璐, 李叶阔. 载介孔二氧化硅纳米囊脂质体增强高强度聚焦超声对肿瘤细胞的杀伤力[J]. 中国医学影像学杂志, 2016, 24: 321–328. DOI: 10.3969/j.issn.1005-5185.2016.05.001 |
| [14] | LIU J, XU J, ZHOU J, ZHANG Y, GUO D, WANG Z. Fe3O4-based PLGA nanoparticles as MR contrast agents for the detection of thrombosis[J]. Int J Nanomedicine, 2017, 12: 1113–1126. DOI: 10.2147/IJN |
| [15] | LI F, ZHI D, LUO Y, ZHANG J, NAN X, ZHANG Y, et al. Core/shell Fe3O4/Gd2O3 nanocubes as T1-T2 dual modal MRI contrast agents[J]. Nanoscale, 2016, 8: 12826–12833. DOI: 10.1039/C6NR02620F |
 2017, Vol. 38
2017, Vol. 38


