2. 第二军医大学长海医院中医科, 上海 200433
2. Department of Traditional Chinese Medicine, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
胃癌是我国高发的恶性肿瘤之一,死亡率高居全球恶性肿瘤的第2位[1]。淋巴转移是影响胃癌不良预后的重要因素之一[2],研究表明20%的黏膜下癌存在淋巴转移[3],但关于淋巴转移的具体机制尚不清楚。本研究通过检测胃癌、癌旁及胃周淋巴结组织中与淋巴管生成相关的血管内皮生长因子(vascular endothelial growth factor,VEGF)-C、VEGF-D及血管内皮生长因子受体3(vascular endothelial growth factor receptor 3,VEGFR-3)的蛋白表达水平,并分析其临床意义,为进一步研究胃癌的淋巴转移机制奠定基础。
1 资料和方法 1.1 一般资料标本来自2016年1月至2016年6月于第二军医大学长征医院行手术治疗的胃癌患者,患者术前均未接受放射治疗、化学治疗、靶向治疗等抗肿瘤治疗。收集同一患者的胃癌原发灶、癌旁组织(距离原发灶边缘≥5 cm,切缘阴性)、淋巴结组织。共收集60例,其中男性44例,女性16例,平均年龄(62.21±11.89)岁。
1.2 免疫组化染色标本采用甲醛固定后用石蜡包埋。石蜡标本切片后进行免疫组化SP法染色。抗VEGF-C、抗VEGF-D、抗VEGFR-3抗体订购自英国Abcam公司。根据Reset细胞和染色强度综合评分法[4]将细胞着色评分与着色细胞数评分相乘,所得综合评分分为4个等级:阴性为0~1分,弱阳性为2~3分,阳性为4~6分,强阳性为>6分。
1.3 胃癌组织幽门螺杆菌(H.pylori)检测采用硼酸亚甲蓝染色方法[5]检测H.pylori。
1.4 统计学处理采用SPSS 17.0软件进行数据分析。数据以x±s表示,计量资料的比较采用t检验或方差分析。检验水准(α)为0.05。
2 结果 2.1 胃癌和癌旁组织中VEGF-C、VEGF-D和VEGFR-3蛋白表达水平如图 1所示,胃癌组织中VEGF-C、VEGF-D蛋白表达水平高于癌旁组织(2.82±1.66 vs 2.13±1.75,4.81±2.30 vs 3.78±1.94;P均 < 0.05)。VEGFR-3蛋白表达水平在胃癌组织和癌旁组织中差异无统计学意义(3.54±3.39 vs 3.38±2.19,P > 0.05)。
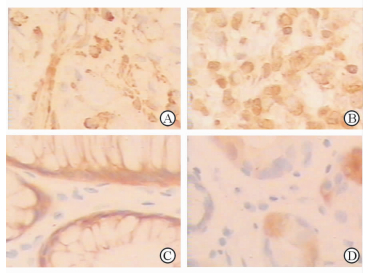
|
图 1 胃癌(A、B)及癌旁组织(C、D)中VEGF-C(A、C)与VEGF-D(B、D)的蛋白表达 VEGF:血管内皮生长因子.免疫组化SP法染色.Original magnification: ×200 |
2.2 胃癌转移淋巴结与未转移淋巴结中VEGF-C、VEGF-D、VEGFR-3蛋白表达水平
胃癌转移的淋巴结组织中VEGF-C、VEGF-D、VEGFR-3的蛋白表达水平分别为3.77±2.43、2.86±1.95、2.55±2.11(n=44),均高于未转移淋巴结中的蛋白表达水平(2.25±2.01、1.98±1.73、0.76±1.13,n=59,P均 < 0.05)。见图 2。
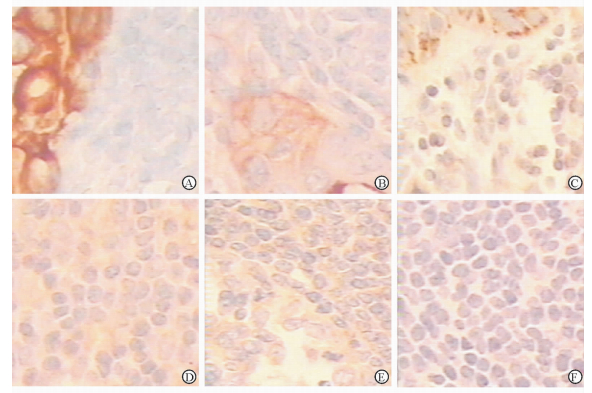
|
图 2 转移淋巴结(A~C)与未转移淋巴结组织(D~F)中VEGF-C(A、D)、VEGF-D(B、E)、VEGFR-3(C、F)的蛋白表达 VEGF:血管内皮生长因子;VEGFR:血管内皮生长因子受体.免疫组化SP法染色.Original magnification: ×200 |
2.3 各临床参数与胃癌组织VEGF-C、VEGF-D、VEGFR-3蛋白表达水平的关系
如表 1所示,不同性别、年龄、肿瘤分化程度、Lauren分型以及肿瘤的浸润深度对VEGF-C、VEGF-D、VEGFR-3表达水平均无明显影响,组间差异均无统计学意义(P均> 0.05)。但在淋巴结转移参数方面,伴有淋巴结转移的胃癌组织中VEGF-C、VEGF-D的蛋白表达水平高于无淋巴结转移的胃癌组织(P均 < 0.05);在H.pylori阳性的胃癌组织中VEGFR-3的蛋白表达水平高于H.pylori阴性的胃癌组织(P < 0.05)。
|
|
表 1 各临床参数与VEGF-C、VEGF-D、VEGFR-3蛋白表达的关系 |
3 讨论
淋巴转移是包括胃癌在内的多种恶性肿瘤转移的重要途径之一。VEGF是肿瘤血管生成的重要调控因子,参与肿瘤的血管生成及侵袭转移等恶性生物学行为[6]。随着研究的不断深入,有研究者发现VEGF还与淋巴管的生成存在密切联系[7]。
VEGF-C、VEGF-D是VEGF家族成员,与淋巴管的生成关系密切[8]。抑制肿瘤细胞分泌VEGF-C可以抑制肿瘤的淋巴管生成[9],VEGF-C、VEGF-D可能会成为治疗肿瘤淋巴转移的靶点[10-11]。VEGFR-3是VEGF-C、VEGF-D的受体,在正常成人组织中VEGFR-3主要局限在淋巴管内皮细胞,然而在多种恶性肿瘤患者中可伴有VEGFR-3的异常升高[12-13]。VEGF-C、VEGF-D与VEGFR-3结合后可促进淋巴管的形成,从而促进肿瘤的淋巴转移。
本研究表明,在转移的淋巴结组织中VEGF-C及VEGF-D、VEGFR-3的蛋白表达水平均高于未转移的淋巴结,在胃癌组织中VEGF-C、VEGF-D高于癌旁组织;进一步的临床参数分析结果显示,伴有淋巴结转移的胃癌组织中VEGF-C、VEGF-D的蛋白表达水平高于未伴有淋巴结转移的胃癌组织,表明VEGF-C及VEGF-D参与胃癌的淋巴转移,其异常高表达是促进胃癌淋巴转移的重要因素。在本研究中VEGFR-3在胃癌组织中并未与VEGF-C、VEGF-D一样高于癌旁组织,这表明除了与VEGFR-3结合外,VEGF-C、VEGF-D可能还通过其他途径参与淋巴转移。
淋巴转移过程会受到多种因素的影响。由于H.pylori感染被列为胃癌的Ⅰ类致癌因素,为了明确H.pylori感染是否对胃癌的淋巴转移有影响,本研究进一步分析了H.pylori阳性胃癌组织与H.pylori阴性胃癌组织中VEGFR-3、VEGF-C及VEGF-D的蛋白表达水平。结果显示,H.pylori感染仅对胃癌组织的VEGFR-3蛋白表达水平有促进作用,还不能认为H.pylori感染与胃癌淋巴转移有关。此外,性别、年龄、肿瘤分化程度等临床参数对胃癌组织中VEGF-C、VEGF-D、VEGFR-3的蛋白表达水平未见明显影响。
综上,VEGF-C、VEGF-D蛋白表达水平在胃癌原发灶和转移淋巴结中增高,且与胃癌的淋巴转移有关。
| [1] | HUNT R H, CAMILLERI M, CROWE S E, EL-OMAR E M, FOX J G, KUIPERS E J, et al. The stomach in health and disease[J]. Gut, 2015, 64: 1650–1668. DOI: 10.1136/gutjnl-2014-307595 |
| [2] | ZHANG J, ZHU Z, SHENG J, YU Z, YAO B, HUANG K, et al. miR-509-3-5P inhibits the invasion and lymphatic metastasis by targeting PODXL and serves as a novel prognostic indicator for gastric cancer[J]. Oncotarget, 2017, 8: 34867–34883. |
| [3] | COBURN N G. Lymph nodes and gastric cancer[J]. J Surg Oncol, 2009, 99: 199–206. DOI: 10.1002/jso.v99:4 |
| [4] | NISHIZAWA T, SUZUKI H. Gastric carcinogenesis and underlying molecular mechanisms:helicobacter pylori and novel targeted therapy[J/OL]. Biomed Res Int, 2015, 2015:794378. doi:10.1155/2015/794378. |
| [5] | 殷正进, 李桂梅, 曹晓卉. 幽门螺杆菌三种检测方法的比较[J]. 诊断病理学杂志, 2015, 22: 647–648. DOI: 10.3969/j.issn.1007-8096.2015.10.022 |
| [6] | BALLA M M, DESAI S, PURWAR P, KUMAR A, BHANDARKAR P, SHEJUL Y K, et al. Differential diagnosis of lung cancer, its metastasis and chronic obstructive pulmonary disease based on serum VEGF, IL-8 and MMP-9[J/OL]. Sci Rep, 2016, 6:36065. doi:10.1038/srep36065. |
| [7] | MORFOISSE F, RENAUD E, HANTELYS F, PRATS A C, GARMY-SUSINI B. Role of hypoxia and vascular endothelial growth factors in lymphangiogenesis[J/OL]. Mol Cell Oncol, 2015, 2:e1024821. doi:10.1080/23723556.2015.1024821. |
| [8] | BUI H M, ENIS D, ROBCIUC M R, MURMI H J, COHEN J, CHEN M, et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD[J]. J Clin Invest, 2016, 126: 2167–2180. DOI: 10.1172/JCI83967 |
| [9] | LEI Y, LI B, TONG S, QI L, HU X, CUI Y, et al. miR-101 suppresses vascular endothelial growth factor C that inhibits migration and invasion and enhances cisplatin chemosensitivity of bladder cancer cells[J/OL]. PLoS One, 2015, 10:e0117809. doi:10.1371/journal.pone.0117809. |
| [10] | WANG C A, JEDLICKA P, PATRICK A N, MICALIZZI D S, LEMMER K C, DEITSCH E, et al. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer[J]. J Clin Invest, 2012, 122: 1895–1906. DOI: 10.1172/JCI59858 |
| [11] | ACHEN M G, MANN G B, STACKER S A. Targeting lymphangiogenesis to prevent tumour metastasis[J]. Br J Cancer, 2006, 94: 1355–1360. DOI: 10.1038/sj.bjc.6603120 |
| [12] | KURENOVA E V, HUNT D L, HE D, FU A D, MASSOLL N A, GOLUBOVSKAYA V M, et al. Vascular endothelial growth factor receptor-3 promotes breast cancer cell proliferation, motility and survival in vitro and tumor formation in vivo[J]. Cell Cycle, 2009, 8: 2266–2280. DOI: 10.4161/cc.8.14.9101 |
| [13] | LI C, FAN J, SONG X, ZHANG B, CHEN Y, LI C, et al. Expression of angiopoietin-2 and vascular endothelial growth factor receptor-3 correlates with lymphangiogenesis and angiogenesis and affects survival of oral squamous cell carcinoma[J/OL]. PLoS One, 2013, 8:e75388. q. |
 2017, Vol. 38
2017, Vol. 38


