流行病学资料表明,射血分数保留心力衰竭(heart failure with preserved ejection fraction,HFpEF)的发病率逐年升高,占心力衰竭总发病率的50%以上,年死亡率高达9%[1],严重影响患者预后。与射血分数减低心力衰竭(heart failure with reduced ejection fraction,HFrEF)相比,HFpEF的临床表现缺乏特异性,常易漏诊和误诊。心导管技术虽然被公认为评价心脏舒张功能的“金标准”[2],但因为是有创操作并且价格昂贵,难以广泛应用。临床多采用超声心动图(ultrasound cardiogram,UCG)血流及组织多普勒成像(tissue Doppler imaging,TDI)技术测定与心脏舒张功能相关的一系列复合指标,然后进行多层逻辑性评估[3]。但是UCG结果受检测设备、受检者自身条件及观察者间差异等影响,数据缺乏客观稳定性。因此迫切需要寻找更加客观、稳定、无创的影像学诊断标准,精准评估心脏舒张功能。近年心脏磁共振(cardiac magnetic resonance,CMR)技术发展迅速,且精确性、稳定性和客观性优于UCG,预计将成为评价心脏舒张功能的重要手段[4]。既往研究多应用CMR评估心脏收缩功能与节段性室壁运动异常[5],采用CMR评价心脏舒张功能的研究甚少。本研究采用易获取的CMR电影成像序列,探讨容积-时间曲线(volume-time curve,VTC)及心肌应变(strain)参数评价HFpEF心脏舒张功能的可行性及准确性。
1 资料和方法 1.1 研究资料HFpEF组:参考2012年欧洲心脏病学会心力衰竭指南[6],续贯纳入2015年9月至2016年9月在我院接受CMR检查的HFpEF患者30例。纳入标准:(1)具有典型心力衰竭症状和体征;NYHA(New York Heart Association)分级≥Ⅱ级;(2)左心室收缩功能正常且左心室无扩大,左心室射血分数(left ventricular ejection fraction,LVEF)≥50%,左心室舒张末期容积指数(left ventricular end-diastolic volume index,LVEDVI) < 97 mL/m2;(3)UCG有舒张功能异常的证据,应用TDI测定左房室瓣口舒张早期血流速度峰值(E)与左房室瓣环舒张早期运动速度(e’)的比值(E/e’)≥15,或TDI测定8 < E/e’ < 15且满足血浆氨基末端B型利钠肽前体(NT-proBNP)>220 pg/mL或B型利钠肽(BNP)>200 pg/mL。对照组:选取同期接受CMR检查的心功能正常者15例,年龄、性别与HFpEF患者相匹配,无胸闷气短的临床症状,NT-proBNP≤220 pg/mL,TDI测定E/e’≤8,LVEF>50%。同一受检者CMR及UCG检查时间间隔小于2周。排除标准:(1)心脏瓣膜病、肥厚性心肌病、限制性心肌病、持续性房颤等;(2)合并未控制的感染、严重的电解质紊乱,合并严重的肺、肝、肾功能不全;(3)妊娠或哺乳期患者;(4)合并有精神疾病或不愿意合作者;(5)有CMR增强扫描禁忌证者(如心脏起搏器或埋藏式心脏复律除颤器植入等)。所有受检者均签署知情同意书。本研究通过医院伦理审批[审批编号:2017-KY-003(K)]。
1.2 UCG检查采用Siemens公司Sequoia 512彩色多普勒超声仪,探头3V2c,探头频率3~8 MHz,测量参数取3个连续的心动周期的平均值。舒张功能指标:于心尖四腔切面,将取样容积置于左房室瓣瓣尖,获取舒张期血流频谱,测量左房室瓣口E及左房室瓣口舒张晚期血流速度峰值(A),计算E/A比值;采用TDI技术获取室间隔、侧壁、下壁、前壁左房室瓣环水平的运动速度,测量4个区域的e’,采用4个区域e’平均值计算E/e’比值。
1.3 CMR扫描与分析采用飞利浦3.0T磁共振成像仪(Philips Healthcare),配备四导联心电触发及呼吸导航监视装置,扫描左心室长轴两腔、三腔、四腔心及系列短轴电影序列。扫描参数:重复时间(repetition time,TR)=3.2,回波时间(echo time,TE)=1.5,翻转角(flip angle,FA)为45°,矩阵为232×219。其中左心室短轴电影序列从心尖至心底无间隔采集,层数10~12层,层厚8 mm。
采用飞利浦LV analysis程序半自动识别左心室内外膜轮廓,自动绘制VTC,采集左心室舒张早期峰值充盈速率(first peak filling rate,PFR1)和舒张早期充盈量(first filling volume,FV1)、舒张晚期峰值充盈速率(second peak filling rate,PFR2)和舒张晚期充盈量(second filling volume,FV2)及总充盈量(total filling volume,FV)。同时利用该软件获取LVEF、左心室舒张末期容积(left ventricular end-diastolic volume,LVEDV)、左心室收缩末期容积(left ventricular end-systolic volume,LVESV)、左心室质量(left ventricular mass,LVM)、左心房容积(left atrial volume,LAV),并计算左心室重构指数(left ventricular remodeling index,LVRI;LVRI=LVM/LVEDV[7])。然后,采用Circle Cardiovascular Imaging CVI42磁共振后处理软件Tissue Tracking模块分析左心室应变,绘制左心室整体应变曲线,计算左心室整体纵向峰值应变(global longitudinal peak strain,GLS)及舒张期整体纵向峰值应变率(global longitudinal peak diastolic strain rate,GDLSR)、整体径向峰值应变(global radial peak strain,GRS)及舒张期整体径向峰值应变率(global radial peak diastolic strain rate,GDRSR)、整体周向峰值应变(global circumferential peak strain,GCS)及舒张期整体周向峰值应变率(global circumferential peak diastolic strain rate,GDCSR)。所有图像分析与测量分别由2位心脏影像主任医师进行。
1.4 统计学处理应用SPSS 21.0软件进行统计学分析。符合正态分布的计量资料以x±s表示,组间比较采用t检验。计数资料以百分比表示,组间比较采用χ2检验。检验水准(α)为0.05。
2 结果 2.1 两组基本资料的比较HFpEF组NYHA分级:Ⅱ级11例(36.7%)、Ⅲ级16例(53.3%)、Ⅳ级3例(10.0%);血浆NT-proBNP水平220~1 000 pg/mL,中位数500 pg/mL。与对照组相比,HFpEF组多合并冠心病、高血压、糖尿病(P < 0.05),e’降低,E/e’比值升高(P < 0.001),其余基线参数差异均无统计学意义。见表 1。
|
|
表 1 研究对象基线临床参数 Tab 1 Baseline clinical characteristics |
2.2 CMR检查结果
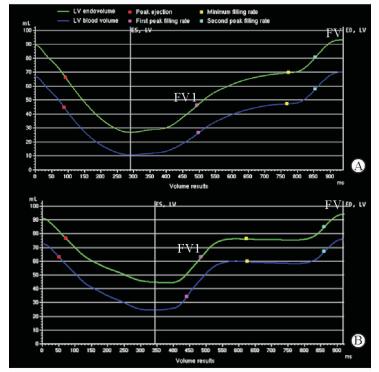
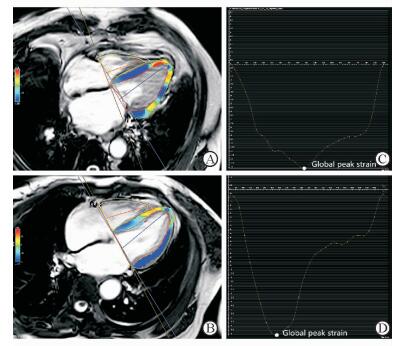
由表 2可见,两组间LVEF、LVEDV、LVESV、LVM差异均无统计学意义(P均>0.05),但是与对照组相比,HFpEF组LVRI升高(P < 0.05)。VTC(图 1)显示,与对照组相比,HFpEF组左心室收缩时间缩短,舒张时间相对延长,平台期缩短,PFR1降低而PFR2升高,PFR1/PFR2比值降低。对照组PFR1/PFR2>1,HfpEF组 < 1,两组差异有统计学意义(P=0.001,表 2)。尤其是对于UCG显示E/A假性正常的HFpEF患者(本研究中有2例,E/A平均值为1.25±0.02),CMR能够准确显示PFR1/PFR2 < 1(2例的PFR1/PFR2平均值为0.678±0.014)。HFpEF组FV1及FV1/FV较对照组均降低(P均 < 0.05,表 2),其中对照组FV1/FV为0.69±0.03(0.60~0.80)、HFpEF组为0.59±0.05(0.55~0.65)。HEpEF组的左心室整体应变应力图及曲线形态发生改变(图 2),伪彩图(图 2A)中左心室侧壁心肌由均一颜色变为多种颜色,代表心肌的应力下降,提示心肌的损伤及僵硬程度;图 2C所示曲线提示左心室整体纵向应变水平均下降,峰值应变降低,应变数值随时间的变化幅度减小。由表 2可见,HFpEF组GLS小于对照组(P < 0.01),而两组GRS、GCS的差异均无统计学意义(P均>0.05);但HFpEF组GDLSR、GDRSR、GDCSR均低于对照组(P均 < 0.05)。
|
|
表 2 HFpEF与对照组CMR参数的比较 Tab 2 Comparison of cardiac magnetic resonance (CMR) parameters between HFpEF and control groups |
|
图 1 左心室VTC Fig 1 Left ventricular (LV) volume-time curve (VTC) Y-axis represents volume (mL), X-axis represents time (ms). A: In the HFpEF group, the LV diastole expanded, and the plateau shortened, with the first peak filling rate increasing and the second peak filling rate decreasing, resulting in a decrease in the first filling volume (FV1), as well as the FV1 to total filling volume (FV) ratio; B: Normal filling rate and filling volume in control group. HFpEF: Heart failure with preserved ejection fraction |
|
图 2 左心室CMR应变曲线示意图 Fig 2 Left ventricular (LV) measurements by tissue-tracking cardiac magnetic resonance (CMR) A, B: LV longitudinal strain in the four-chamber views at the end of LV systole, and the color changes of the pseudo in A indicated the myocardial damage in an HFpEF patient; C, D: The strain curves showed changes of GLS graph over time (C: The peak of GLS was decreased in an HFpEF patient; D: Normal GLS in control group). HFpEF: Heart failure with preserved ejection fraction; GLS: Global longitudinal peak strain |
3 讨论
本研究通过CMR检测显示HFpEF患者LVRI增加,左心室VTC显示左心室舒张期充盈速率PFR1/PFR2比值降低、FV1和FV1/FV比值降低,反映心肌舒张力的新指标GDLSR、GDRSR及GDCSR均降低;在LVEF未表现异常的情况下,HFpEF患者已经出现GLS降低,提示应变参数GLS较传统指标LVEF能够更早地提示心肌收缩功能的下降。
本研究纳入的研究对象特点为高龄女性居多,多合并有冠心病、高血压、糖尿病等基础疾病,与既往研究中HFpEF的危险因素相似[8-9],这些患者往往存在高血压及年龄相关性的心脏形态结构改变。CMR电影成像序列后处理采用左心室短轴逐层叠加算法,不受心脏几何形态改变的影响,保证了心室容积测量结果的精确性[10]。本研究发现HFpEF患者LVM增高,但仍在我国正常人均值范围[11]内,且与对照组相比差异无统计学意义。进一步采用LVEDV校正获得LVRI后,则表现出组间差异有统计学意义(P < 0.05)。Buakhamsri等[12]研究发现,在扩张型心肌病及肥厚型心肌病中,LVRI可以提示心脏舒张功能障碍。本研究进一步证实了LVRI可作为判断HFpEF患者左心室舒张功能的指标。
本研究探讨了VTC能否准确反映HFpEF患者心室主动松弛能力的下降。既往研究显示,冠心病及心肌病可引起左心室充盈模式的改变[13-14]。通过CMR获得的VTC能够描述左心室容积在整个心动周期内的动态变化过程。本研究发现,与对照组相比HFpEF患者PFR1降低,PFR2升高,PFR1/PFR2降低或倒置,提示HFpEF患者左心室顺应性异常及僵硬度增加,心肌刚性、弹性回缩力下降,所以早期充盈速率降低。同类型研究表明正常人FV1/FV约为0.60~0.80[15],与本研究对照组数值0.69±0.03(0.60~0.80)相似,而本研究中HFpEF组FV1/FV为0.59±0.05(0.55~0.65),较对照组降低,提示HFpEF患者左心室舒张早期充盈受损。因为舒张早期充盈是心室的主动充盈,由房室间压力差引起,早期充盈量减少,晚期充盈量代偿性增加。通过UCG测量左房室瓣E峰和A峰的缺陷是在二维平面获取参数,而心腔是三维结构,所以E/A无法区别舒张功能正常和假性正常的患者,故需要测量E/e’进一步判断。CMR能够实现三维测量舒张PFR1及PFR2,PFR1/PFR2比值在对照组中大于1,在假性正常的患者中出现倒置,可见PFR1/PFR2能够较敏感地提示左心室舒张功能的改变。
应变成像是TDI新技术,包括纵向应变、径向应变和周向应变,既往研究重点集中在心肌病[16],尚无采用CMR应变技术评价HFpEF患者心脏功能的报道。静息状态下,HFpEF患者LVEF正常,但是其心肌收缩性能是否受损仍存在争议。Carluccio等[17]的研究显示,使用超声TDI技术获得GLS是判断左心室收缩功能的敏感指标。PARAMOUNT研究运用UCG斑点追踪技术,发现在LVEF正常的情况下,HFpEF组GLS降低,提示收缩功能减退[18]。上述两个研究均采用超声技术。本研究通过CMR组织追踪技术对运动心肌进行标记,进一步检测HFpEF患者的收缩功能。CMR组织追踪技术能够三维立体还原左心室真实的运动和形变,优于UCG二维斑点追踪技术,与传统MRI标记技术(tagging)相比,大大缩短扫描时间,适用于心力衰竭伴呼吸困难的患者。结果显示HFpEF患者的GLS在左心室GRS和GCS未见明显改变的情况下降低,提示HFpEF患者的左心室长轴纵向的心肌缩短能力下降,而径向及周向的心肌缩短能力保留。因此GLS能够提示早期左心室收缩功能损伤,有利于临床医师早诊断、早干预。
此外,本研究还发现与对照组相比,HFpEF组左心室GDLSR、GDRSR及GDCSR均下降,其中以GDCSR下降最为显著。左心室舒张期峰值应变率是心脏在一个心动周期的舒张过程中发生形变的最大速率,不受心肌整体位移和周围组织牵拉的影响,可准确反映心肌运动的细微变化。既往有研究使用CMR纵向弛豫时间定量成像(T1 mapping)技术发现GDCSR的降低与心肌内弥散性纤维化有关[19-20],本研究采用CMR常规电影技术在不增加扫描序列前提下,同样发现GDCSR在HFpEF患者中降低,心肌舒张期形变的能力在纵向、径向、周向3个方向上均减弱。说明左心室舒张期峰值应变率能够反映左心室的舒张功能,有望成为诊断HFpEF的有效指标。
本研究的局限性:(1)本研究是单中心研究,样本量较小,有待进一步扩大数据量并完善随访数据;(2)CMR受心律及呼吸影响较大,因而舒张性心力衰竭伴心房颤动的患者不在研究之列。
| [1] | AZIZ F, THAZHATAUVEETIL-KUNHAHAMED L A, ENWELUZO C, ZAEEM M. Heart failure with preserved EF:a bird eye view[J]. JNMA J Nepal Med Assoc, 2013, 52: 405–412. |
| [2] | HUSAINS, PAMBOUKIAN S V, TALLAJ J A, MCGIFFIN D C, BOURGE R C. Invasive monitoring in patients with heart failure[J]. Curr Cardiol Rep, 2009, 11: 159–225. DOI: 10.1007/s11886-009-0024-x |
| [3] | NAGUEH S F, SMISETH O A, APPLETON C P, BYRD B F 3rd, DOKAINISH H, EDVARDSEN T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography:an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging[J]. Eur Heart J Cardiovasc Imaging, 2016, 17: 1321–1360. DOI: 10.1093/ehjci/jew082 |
| [4] | WESTENBERG J J. CMR for assessment of diastolic function[J]. Curr Cardiovasc Imaging Rep, 2011, 4: 149–158. DOI: 10.1007/s12410-011-9070-z |
| [5] | AFSHIN M, BEN AYED I, PUNITHAKUMAR K, LAW M, ISLAM A, GOELA A, et al. Regional assessment of cardiac left ventricular myocardial function via MRI statistical features[J]. IEEE Trans Med Imaging, 2014, 33: 481–494. DOI: 10.1109/TMI.2013.2287793 |
| [6] | McMURRAY J J, ADAMOPOULOS S, ANKER S D, AURICCHIO A, BÖHM M, DICKSTEIN K, et al. ESC Committee for Practice Guidelines.ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012:The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC[J]. Eur J Heart Fail, 2012, 14: 803–869. DOI: 10.1093/eurjhf/hfs105 |
| [7] | CHEN Y Z, QIAO S B, HU F H, YUAN J S, YANG W X, CUI J G, et al. Left ventricular remodeling and fibrosis:sex differences and relationship with diastolic function in hypertrophic cardiomyopathy[J]. Eur J Radiol, 2015, 84: 1487–1492. DOI: 10.1016/j.ejrad.2015.04.026 |
| [8] | WOOD P, PIRAN S, LIU P P. Diastolic heart failure:progress, treatment challenges, and prevention[J]. Can J Cardiol, 2011, 27: 302–310. DOI: 10.1016/j.cjca.2011.02.008 |
| [9] | YANCY C W, LOPATIN M, STEVENSON L W, DE MARCO T, FONAROW G C. ADHERE Scientific Advisory Committee and Investigators. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function:a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database[J]. J Am Coll Cardiol, 2006, 47: 76–84. DOI: 10.1016/j.jacc.2005.09.022 |
| [10] | 蔡仁慧, 赵新湘, 孙林. 心脏磁共振技术在心肌梗死后心室重构中的应用[J]. 中国循环杂志, 2015, 30: 926–928. DOI: 10.3969/j.issn.1000-3614.2015.09.026 |
| [11] | 陆敏杰, 赵世华, 蒋世良, 尹刚, 张岩, 凌坚, 等. 中国人心脏房室腔内径及左右心室功能正常参数的MRI研究[J]. 中华放射学杂志, 2011, 45: 924–928. DOI: 10.3760/cma.j.issn.1005-1201.2011.10.008 |
| [12] | BUAKHAMSRI A, POPOVIC Z B, LIN J, LIM P, GREENBERG N L, BOROWSKI A G, et al. Impact of left ventricular volume/mass ratio on diastolic function[J]. Eur Heart J, 2009, 30: 1213–1234. DOI: 10.1093/eurheartj/ehp084 |
| [13] | RODRÍGUEZ-GRANILLO G A, MEJÍA-CAMPILLO M, ROSALES M A, BOLZÁN G, INGINO C, LÍPEZ F, et al. Left ventricular filling patterns in patients with previous myocardial infarction measured by conventional cine cardiac magnetic resonance[J]. Int J Cardiovasc Imaging, 2012, 28: 795–801. DOI: 10.1007/s10554-011-9883-5 |
| [14] | CHENX, HU H, QIAN Y, SHU J. Relation of late gadolinium enhancement in cardiac magnetic resonance on the diastolic volume recovery of left ventricle with hypertrophic cardiomyopathy[J]. J Thorac Dis, 2014, 6: 988–994. |
| [15] | 任宇婧, 胡红杰, 钱玉娥, 陈晓荣, 刘华锋, 赵博文. 采用心脏MRI时间容量曲线评价肥厚型心肌病左心室舒张功能的可行性研究[J]. 中华放射学杂志, 2012, 46: 896–901. DOI: 10.3760/cma.j.issn.1005-1201.2012.10.006 |
| [16] | 江鸿, 智光, 阎国辉, 侯海军, 周肖, 徐勇. 肥厚型心肌病患者左室心肌收缩期应变率的速度向量成像定量分析[J]. 第二军医大学学报, 2008, 29: 390–394. DOI: 10.3321/j.issn:0258-879X.2008.04.010 |
| [17] | CARLUCCIOE, BIAGIOLI P, ALUNNI G, MURRONE A, LEONELLI V, PANTANO P, et al. Advantages of deformation indices over systolic velocities in assessment of longitudinal systolic function in patients with heart failure and normal ejection fraction[J]. Eur J Heart Fail, 2011, 13: 292–302. DOI: 10.1093/eurjhf/hfq203 |
| [18] | KRAIGHER-KRAINER E, SHAH A M, GUPTA D K, SANTOS A, CLAGGETT B, PIESKE B, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction[J]. J Am Coll Cardiol, 2014, 63: 447–456. DOI: 10.1016/j.jacc.2013.09.052 |
| [19] | NTUSIN A, PIECHNIK S K, FRANCIS J M, FERREIRA V M, RAI A B, MATTHEWS P M, et al. Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis-a clinical study using myocardial T1-mapping and extracellular volume quantification[J/OL]. J Cardiovasc Magn Reson, 2014, 16:21.doi:10.1186/1532-429x-16-21. |
| [20] | NTUSI N A B, PIECHNIK S K, FRANCIS J M, FERREIRA V M, MATTHEWS P M, ROBSON M D, et al. Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis:insights from CMR T1 mapping[J]. JACC Cardiovasc Imaging, 2015, 8: 526–536. DOI: 10.1016/j.jcmg.2014.12.025 |
 2017, Vol. 38
2017, Vol. 38


