2. 第二军医大学长海医院心血管内科, 上海 200433;
3. 第二军医大学长海医院胸心外科, 上海 200433;
4. 上海锦葵医疗器械有限公司研发部, 上海 201103
2. Department of Cardiovasology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
3. Department of Cardiothoracic Surgery, Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
4. Research and Development Department, Shanghai Jinkui Medical Instruments Co. Ltd., Shanghai 201103, China
心房颤动(房颤)是诱发脑卒中的危险因素,对于非瓣膜性房颤患者,约90%的血栓栓子来源于左心耳[1],左心耳封堵疗法可以通过封闭“血栓窝点”来预防脑卒中。近年来经外周血管左心耳封堵疗法发展迅速,但由于封堵器的骨架皆由金属材料制作而成,且手术过程需要房间隔穿刺及多次释放回收来找到理想位置,因此会出现较多的围手术期并发症,如心包填塞、大出血、金属封堵器压迫回旋支、封堵器相关的血栓形成以及随心脏反复跳动侵蚀周围组织等[2-5]。为了避免因金属长期留置体内而造成的并发症,我们与上海锦葵医疗器械有限公司合作,使用可吸收材料制作了左心耳封堵器,拟尝试经胸逆向途径进行左心耳封堵。本研究通过在离体犬心脏标本上进行初步的封堵实验,探讨可吸收左心耳封堵器的优化设计以及经胸逆向封堵左心耳术式的可行性,为下一步动物实验奠定基础。
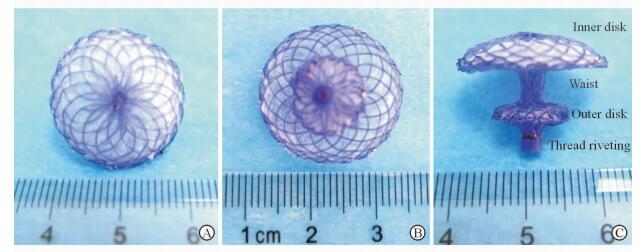
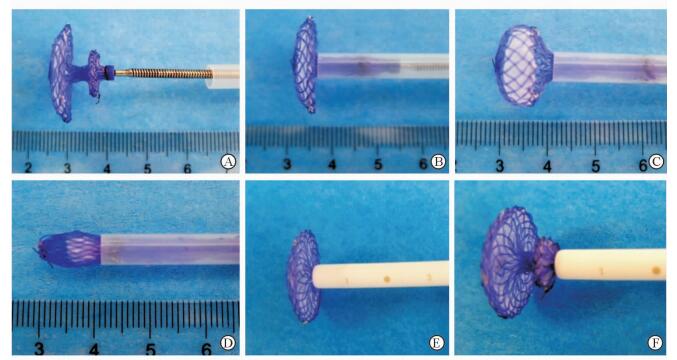
1 材料和方法 1.1 可吸收左心耳封堵器和输送系统的制作封堵器由可吸收材料聚对二氧环己酮(polydioxanone,PDO)丝材、聚乳酸(poly-L-lactic acid,PLA)无纺布和聚乙醇酸(polyglycolic acid,PGA)缝线制作并经热定型而成,呈不对称的双盘状(图 1)。其中PDO丝材作为封堵器的骨架,PLA无纺布作为阻挡血流的隔膜,由PGA缝线缝制。参照文献[6]中的左心耳解剖参数,确定封堵器心耳口盘片(内盘)大小和腰部高度。封堵器心耳外侧盘面(外盘)的中央设计为带内螺纹铆样结构,以与输送系统推送杆连接。参照文献[6]设计封堵器的输送系统,包括带刻度输送鞘、匹配的扩张鞘、预载鞘和推送杆。输送鞘表面附有刻度,扩张鞘头端为平滑过渡设计,头端仅露出输送鞘约0.8 cm (图 2)。在体外条件下,可吸收封堵器可以通过输送系统顺利收入、输送、释放和回收(图 3)。封堵器和输送系统委托上海锦葵医疗器械有限公司制作。
|
图 1 可吸收左心耳封堵器外观 Fig 1 Appearance of absorbable left atrial appendage (LAA) occluder A: LAA occluder inner disk view; B: LAA occluder outer disk view; C: LAA occluder side view |

|
图 2 输送系统 Fig 2 Delivery system A: Delivery system including delivery sheath, expanding sheath, preloading sheath and pushing rod; B: The head of expanding sheath with scale emerging from delivery sheath for about 0.8 cm |
|
图 3 封堵器的输送、释放以及回收 Fig 3 Delivery, release and retrieve of a occluder A: The internal thread riveting in the center of left atrial appendage (LAA) lateral surface was jointed by the push cable of delivery system; B-D: The LAA occluder was stuffed and delivered in preload sheath; E, F: The LAA occluder was released and retrieved by delivery sheath |
1.2 封堵器力学性能检测
在体外37 ℃水浴条件下,连接封堵器尾端螺纹铆与推送杆,再将封堵器收入鞘管内并保留15 min后释放,观察封堵器释放后恢复原状的程度(比较内盘面直径的恢复程度)。模拟完成封堵器被收入、输送、释放、回收的过程,观察鞘管大小、输送是否顺利,记录尾部螺纹铆能承受的有效输送次数,判断其弹性性能。
1.3 离体犬心脏左心耳封堵术取6只健康杂种犬(体质量15~18 kg, 雌雄不限,由上海甲干生物科技有限公司提供)的心脏标本[质量(140±15) g],封堵方法同文献[6]。将标本置于操作台上,左侧朝上以充分暴露左心耳,直视下选择左心耳外侧纵轴上中1/3中央作为穿刺部位,围绕穿刺点用4-0 Prolene线制作缝合荷包。穿刺成功后,用短泥鳅导丝建立左心耳外-穿刺点-左心耳-左心房-左心室半轨道,沿轨道送入输送鞘,退出扩张鞘,保留输送鞘头端1.0~1.5 cm于左心耳内。将载有封堵器的预载鞘与输送鞘尾端连接,送入封堵器,待封堵器心耳内盘释放后回拉推送杆,感觉到有阻力提示左心耳口被封堵,固定推送杆并稍微后退输送鞘,释放封堵器心耳外盘,逆时针旋转以完全释放封堵器。缝合荷包,完成封堵过程。
1.4 术后解剖心脏封堵术完成后,观察封堵器心耳外盘的位置,随后解剖心脏。剪除心脏的右心室及右心房,保留肺静脉,剖开左心室,沿左心室流入道剪开房间隔,暴露左心耳开口,观察封堵器内盘的位置、是否牢固、是否影响肺静脉开口和左房室瓣启闭等。
2 结果 2.1 左心耳封堵器及输送系统性能可吸收封堵器呈不对称双盘状,内盘直径有20、22 mm两种规格,腰高8 mm,外盘直径10 mm,双侧盘面缝上PLA无纺布以阻挡血流,尾端设计为铆样结构,内有螺纹可与推送杆牢固连接。输送系统包括14F输送鞘、匹配的扩张鞘、预载鞘和推送杆,总长50 cm,可利用输送鞘外表面刻度掌控鞘管进入左心耳的深度,扩张鞘头端仅露出输送鞘约0.8 cm,不会损伤心脏内部结构。体外实验证实,封堵器可以顺利经14F鞘管收入、输送和释放;在鞘管内保留15 min释放,即刻可恢复到原状96%的程度;尾部螺纹铆能承受(5.20±0.75)次的有效输送释放而不被毁损,具备较好的超弹性性能。
2.2 封堵结果6只心脏标本全部封堵成功,封堵器释放以后,位置良好;牵拉外盘尾部铆,封堵器不移位。
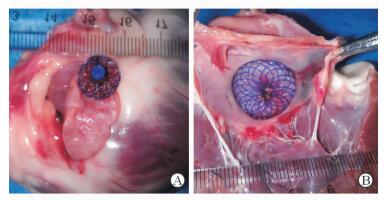
2.3 术后解剖结果解剖心脏发现,内盘进入左心耳口少许,并稳定位于左心耳口正中,与左下、左上肺静脉开口及左房室瓣距离2 mm以上,不影响左上、左下肺静脉的血液回流和左房室瓣的功能,内盘面中心与左心耳口中心大致重合,外盘附着在左心耳外上1/3部位,能够保证封堵器不会脱落(图 4)。撤去封堵器见穿刺点位置正对左心耳开口中央,且处于缝合荷包的中心。
|
图 4 封堵术完成后封堵器位置 Fig 4 Location of occluder after occlusion process A: Left atrial appendage (LAA) outer disk view, the outer disk attaching the upper 1/3 of LAA; B: LAA inner view, the occluder location was good, LAA ostium was over distracted moderately, the distances from LAA ostium edge to left superior pulmonary vein, left inferior pulmonary vein and mitral valve edge were all > 2 mm, and blood flow was not affected |
3 讨论
封闭左心耳“血栓窝点”的方法有外科切除、套扎术、经皮封堵疗法等。外科切除左心耳仅适合在心脏外科手术的同时进行,切除后容易遗留残根[7],缝合分离失败率较高,而且其后血栓形成的风险会上升。经胸腔镜左心耳套扎术操作复杂,学习曲线长,术后有一定的松解率,应用受限[8-9]。经皮封堵疗法近年来发展迅速,但围手术期并发症较多[2-5],且经皮左心耳封堵术对患者左心耳的解剖形态有一定要求。人类左心耳的解剖形状多样,有学者将其归纳为多种形状,如鸡翅样、风向标样、菜花样、仙人掌样等[10]。目前,左心耳封堵器样式主要有“活塞式”(如Watchman封堵器[11])和“覆盖式”(如Amplatzer Cardiac Plug装置[12]),适用于左心耳开口相对较规则、呈圆形或类圆形、管腔有一定深度的患者。但在临床中,笔者发现开口较大者封堵器易脱落,开口较浅者难以寻找合适锚定区域,开口狭长者需要使用较大封堵盘以求全部覆盖开口,为避免封堵盘离肺静脉开口和左房室瓣距离过近导致肺静脉血流和瓣膜启闭受影响,需要经食管超声反复测量以实现封堵器精确定位,这会增加封堵器释放回收次数和操作时间,可能引发心包填塞等并发症。此外,经皮左心耳封堵术采用外周静脉为置入途径,与房间隔缺损封堵术类似,至少需要9~14F的输送鞘,将会增加周围血管并发症的可能性。
封堵器的作用在于为左心耳的闭合提供一座临时“桥梁”,其作用存在时间窗。封堵器植入人体后,周围的组织向封堵器表面生长并完成内皮化,此时的左心耳已经由自身组织闭合,封堵器也就失去了存在的价值。理想的封堵器应在完成“桥梁”作用后可被机体吸收,不再留存体内,让左心耳完成“生理闭合”,以消除因封堵器永久存留体内造成远期并发症的隐患。因此,寻找能让左心耳完成“生理闭合”的可吸收封堵器并通过改良封堵术式拓宽封堵疗法的适应证,有着重要的意义。本着“生理闭合”左心耳的理念,依照前期获得的犬左心耳解剖资料[6],本研究采用生物可吸收材料制作了左心耳封堵器。封堵器呈双盘状,内侧直径为20~22 mm,腰高8 mm,外盘直径皆为10 mm。手术方式采用经胸逆向途径,即在胸骨左缘第4~5肋间靠近脊柱侧小切口开胸,剪开心包,暴露左心耳,围绕心耳外侧上1/3部分的中心制作荷包,然后在荷包中心穿刺,建立半轨道,完成经胸逆向左心耳封堵术。内盘封堵心耳口,外盘贴附于心耳外,保证封堵器不会脱落。本研究采用经胸逆向左心耳封堵术式进行了离体动物实验,均成功实现封堵,封堵器位置良好。解剖发现封堵器内盘位于心耳口内,使心耳开口呈适度过撑状态,不影响肺静脉的血液回流和左房室瓣的功能。
第二军医大学长海医院心血管内科已经研发设计出可吸收房间隔缺损封堵器和室间隔缺损封堵器并完成临床前评价[13-14],其制作材料与本研究所使用的材料相同,动物实验显示封堵器最终会被自身内皮细胞覆盖,且封堵器在6个月左右的时间内可以基本完成降解,被机体吸收,最后由自身组织修复缺损。据此,我们推测可吸收左心耳封堵器在相同时间内也可基本完成降解,并实现左心耳的“生理闭合”。可吸收材料均为高分子材料,相比金属质量更轻,张力更小,不会对回旋支产生压迫作用。
相较于经皮左心耳封堵术,经胸小切口左心耳封堵术的优势在于适应证较宽。由于采用逆向途径术式,只需选择合适大小的封堵器,让内盘进入心耳口使之呈适度过撑状态,即可完成有效封堵,封堵器外盘贴附于心耳外,封堵器不会脱落,因此对左心耳形状没有要求,各种形态的左心耳皆可以实现逆向封堵。
本研究仍存在一定的缺陷:首先,相较于经导管术式来说,小切口开胸创伤较大,术后恢复时间较长;其次,术后围手术期抗凝的策略有待进一步研究。我们设想在小切口开胸的动物实验取得成功以后,拟通过胸腔镜来进行手术,可进一步减少创伤,缩短恢复时间;术后抗凝可以参照外科换瓣术后抗凝经验,避免术后抗凝引起大出血等并发症。
本研究结果表明可吸收左心耳封堵器的设计是合理的,与实验犬心脏解剖有较好的匹配度,实现经胸小切口逆向途径封堵左心耳是可行的。下一步我们将进行动物实验研究,来评价可吸收封堵器经胸逆向封堵左心耳的可行性、安全性和有效性。
| [1] | BLACKSHEAR J L, ODELL J A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation[J]. Ann Thorac Surg, 1996, 61: 755–759. DOI: 10.1016/0003-4975(95)00887-X |
| [2] | LAM Y Y. A new left atrial appendage occluder (Lifetech LAmbre Device) for stroke prevention in atrial fibrillation[J]. Cardiovasc Revasc Med, 2013, 14: 134–136. DOI: 10.1016/j.carrev.2013.04.003 |
| [3] | WHITLOCK R P, HEALEY J S, HOLMES D R. Left atrial appendage occlusion debate revisited[J]. Circulation, 2015, 131: 756–761. DOI: 10.1161/CIRCULATIONAHA.114.008840 |
| [4] | SCHROETER M R, DANNER B C, HUNLICH M, SCHILLINGER W. Uncommon delayed and late complications after percutaneous left atrial appendage closure with Amplatzer Cardiac Plug[J]. Clin Res Cardiol, 2014, 103: 285–290. DOI: 10.1007/s00392-013-0648-0 |
| [5] | CRUZ-GONZALEZ I, YAN B P, Lam Y Y. Left atrial appendage exclusion:state of the art[J]. Catheter Cardiovasc Interv, 2010, 75: 806–813. DOI: 10.1002/ccd.22344 |
| [6] |
储国俊, 朱玉峰, 黄新苗, 吴弘, 章伟, 阚通, 等. 经胸左心耳反向封堵术的器械研制和体外动物实验[J]. 第二军医大学学报, 2015, 36: 1045–1050.
CHU G J, ZHU Y F, HUANG X M, WU H, ZHANG W, KAN T, et al. Transthoracic left atrial appendage reverse occlusion:instrument development and in vitro animal experiment[J]. Acad J Sec Mil Med Univ, 2015, 36: 1045–1050. DOI: 10.3724/SP.J.1008.2015.01045 |
| [7] | HEALEY J S, CRYSTAL E, LAMY A, TEOH K, SEMELHAGO L, HOHNLOSER S H, et al. Left Atrial Appendage Occlusion Study (LAAOS):results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke[J]. Am Heart J, 2005, 150: 288–293. DOI: 10.1016/j.ahj.2004.09.054 |
| [8] | AILAWADI G, GERDISCH M W, HARVEY R L, HOOKER R L, DAMIANO R J Jr, SALAMON T, et al. Exclusion of the left atrial appendage with a novel device:early results of a multicenter trial[J]. J Thorac Cardiovasc Surg, 2011, 142: 1002–1009. DOI: 10.1016/j.jtcvs.2011.07.052 |
| [9] | STONE D, BYRNE T, PERSHAD A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation[J]. Catheter Cardiovasc Interv, 2015, 86: 121–127. DOI: 10.1002/ccd.25065 |
| [10] | SU P, MCCARTHY K P, HO S Y. Occluding the left atrial appendage:anatomical considerations[J]. Heart, 2008, 94: 1166–1170. DOI: 10.1136/hrt.2006.111989 |
| [11] | REDDY V Y, HOLMES D, DOSHI S K, NEUZIL P, KAR S. Safety of pereutaneous left atrial appendage closure:results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registrer[J]. Circulation, 2011, 123: 417–424. DOI: 10.1161/CIRCULATIONAHA.110.976449 |
| [12] | PARK J W, BETHENCOURT A, SIEVERT H, SANTORO G, MEIER B, WALSH K, et al. Left atrial appendage closure with Amplatzer Cardiac Plug in atrial fibrillation:initial European experience[J]. Catheter Cardiovasc Interv, 2011, 77: 700–706. DOI: 10.1002/ccd.v77.5 |
| [13] | ZHU Y F, HUANG X M, CAO J, HU J Q, BAI Y, JIANG B H, et al. Animal experimental study of the fully biodegradable atrial septal defect (ASD) occluder[J/OL]. J Biomed Biotechnol, 2012, 2012:735989. doi:10.1155/2012/735989. |
| [14] | HUANG X M, ZHU Y F, CAO J, HU J Q, BAI Y, JIANG B H, et al. Development and preclinical evaluation of a biodegradable ventricular septal defect occluder[J]. Catheter Cardiovasc Interv, 2013, 81: 324–330. DOI: 10.1002/ccd.v81.2 |
 2017, Vol. 38
2017, Vol. 38


