眼局部组织具有特有的高敏感性和独特的生理功能,使得传统药物剂型如滴眼液[1-2]的生物利用度降低[3]。瞬目、泪液分泌及鼻泪管流失又使得滴眼液从眼表清除迅速[4-5]。当局部给药于眼部后,仅有5%的药物可以穿过角膜到达眼内部组织[1, 6-8]。第二军医大学药学院研究人员使用卡波姆和羟丙甲基纤维素作为主要凝胶基质,盐酸左氧氟沙星为载体药物成功制作了盐酸左氧氟沙星眼用即型凝胶。该凝胶给药前制剂为液体,使用时可以像市售滴眼液一样滴入结膜囊内,在眼部环境下液态凝胶发生相转变为半固体状态的凝胶,可以在眼表停留较长时间。他们对该凝胶的体外释放及在兔眼内的药物消除半衰期进行了研究,体外释放度测定结果显示该凝胶4.5 h时释药约80%,8.0 h时释药约94%,释放药物平缓,具有良好的缓释特性[9]。兔眼内药物消除半衰期研究发现该凝胶的消除半衰期可以达到118.76 min,与文献报道的市售滴眼液的半衰期(约60 min)相比显著延长,可以在较长时间内维持较高房水药物浓度水平[10]。
基于以上实验研究,我们猜想该凝胶是否可以通过药物缓释及维持房水较高药物浓度促进眼内药物吸收,与同药物浓度滴眼液比较是否能更显著地改善临床症状?本实验首次探讨盐酸左氧氟沙星眼用即型凝胶治疗兔眼角膜炎的疗效。
1 材料和方法 1.1 主要材料盐酸左氧氟沙星眼用即型凝胶由第二军医大学药学院药剂学教研室高静副教授惠赠;盐酸左氧氟沙星滴眼液(国药准字H20020106,生产批号:12081301);无菌磷酸盐缓冲液(PBS)购自上海远慕生物科技有限公司;金黄色葡萄球菌菌液(ATCC 25923)购自上海顺勃生物工程技术有限公司。
1.2 动物准备健康新西兰大白兔65只购自上海甲干生物科技有限公司[动物许可证号:SCXK(沪)2010-0028],体质量2.0~2.5 kg,雌雄各半,环境温度(25.0±0.5)℃,控制湿度(55±5)%,1只/笼,12 h昼夜交替饲养,自由饮水摄食,水及食物供给充足。每只兔于实验开始前先适应动物房环境7 d,排除应激因素干扰。实验前24 h用裂隙灯显微镜观察每只兔双眼的结膜、角膜及附属器情况,若存在至少1只眼异常则可排除整只兔,最后有60只兔经检查均合格用于本实验。
1.3 动物模型准备选取60只健康新西兰大白兔,无菌操作下给予左眼角膜基质内注射10 μL ATCC 25923,含105个菌落形成单位(colony-forming units,CFU)。接种24 h时对兔眼进行裂隙灯显微镜观察并进行相应的指标评分。临床观察指标为分泌物、球结膜充血水肿、前方积脓、基质浸润、基质水肿,每项观察指标按严重程度分为4个等级,分别给予0~4分[11-12];采用双盲法由2位观察者同时观察,2人所得评分之和除以2为最后总得分[13-15]。其中48只兔眼炎症评分介于7~9分之间,均出现畏光流泪,眼睑痉挛,少量分泌物,结膜水肿充血,角膜表面形成直径约4 mm的灰白色浸润灶;12只兔眼炎症评分介于17~20分之间,畏光流泪,眼睑痉挛症状严重,大量分泌物致眼睑粘连,结膜高度水肿充血,大量前房积脓,角膜表面形成8~11 mm白色混浊灶。选取炎症反应相近的48只兔进行下一步实验研究。
1.4 给药方法及处理采用完全随机设计方法将兔眼分为4组,每组12只眼,分别为接种菌24 h基线组、PBS空白对照组、盐酸左氧氟沙星滴眼液组、盐酸左氧氟沙星眼用即型凝胶组。
基线组兔眼于细菌接种后24 h时行裂隙灯显微镜观察并进行相应的指标评分,所有观察结束后以静脉空气栓塞法处死该组实验兔;采用完全随机设计方法选取6只眼摘取眼球,将眼球上携带的软组织剔除,立刻用4%甲醛固定眼球防止组织细胞自溶及腐败,同时也防止组织中的酶对蛋白质的分解作用,4%甲醛固定24 h后制作成石蜡切片,H-E染色,重点观察角膜的炎症情况。另外6只眼用眼科镊和手术刀片分离角膜,用无菌生理盐水冲洗后,加入液氮迅速研磨粉碎组织,放入装有1 mL无菌生理盐水的组织研磨器中研磨匀浆,同上述ATCC 25923培养、稀释及计数方法相同,取匀浆液以10倍浓度梯度稀释后接种于牛肉膏蛋白胨培养基中,37℃培养24 h后进行菌落形成单位计数[16]。其他实验组在分组完成后随即开始给药,于每日的8:00、14:00、20:00给药,每次50 μL,连续治疗6 d,于最后一次治疗后2 h进行相应指标评分、角膜病理及角膜细菌菌落计数观察。
1.5 统计学处理数据采用SPSS 18.0软件进行分析,各组角膜临床炎症评分和角膜细菌菌落计数值比较均使用完全随机设计资料的方差分析(analysis of variance,ANOVA),即F检验。检验水准(α)为0.05。
2 结果 2.1 形态学观察及临床评分接种细菌24 h时各组角膜中央形成大小约5 mm的白色菌斑,出现明显细菌性角膜炎临床症状:畏光流泪,眼睑痉挛,结膜充血水肿,结膜囊内白色脓性分泌物,角膜基质浸润及肿胀(图 1A)。

|
图 1 兔眼裂隙灯照相 Fig 1 Slit-lamp photograph of rabbit's eyes |
第7天时,PBS空白对照组兔眼的细菌性角膜炎临床表现进一步加重:菌斑增大、角膜基质浸润及水肿加重,部分眼出现前房积脓(图 1B); 盐酸左氧氟沙星滴眼液组(图 1C)及盐酸左氧氟沙星眼用即型凝胶组(图 1D)兔眼细菌性角膜炎症状明显减轻:角膜菌斑缩小、结膜充血水肿减轻、角膜基质浸润及水肿减轻、角膜透明度增加。
接种细菌24 h时,4组兔眼角膜评分差异无统计学意义(P=0.99);第7天时,盐酸左氧氟沙星眼用即型凝胶组和盐酸左氧氟沙星滴眼液组与PBS空白对照组比较,差异均有统计学意义(P=0.00),盐酸左氧氟沙星眼用即型凝胶组角膜评分小于盐酸左氧氟沙星滴眼液组,但两者差异无统计学意义(P=0.217)。见表 1。
|
|
表 1 各组临床指标观察评分 Tab 1 Clinical scores of each group |
2.2 病理检查
正常眼角膜上皮完整,角膜基质纤维排列有序,无炎症细胞浸润,角膜内皮细胞可见;前房无炎性渗出,房角开放,无炎症细胞浸润。24 h基线组兔眼角膜上皮及基质层肿胀,特别是注射部位即基质的上1/3深度结构紊乱,整个角膜大量炎症细胞浸润。第7天时, PBS空白对照组兔眼角膜上皮及基质明显水肿,大量炎症细胞浸润,角膜上皮缺损,基质层结构紊乱; 盐酸左氧氟沙星滴眼液组角膜上皮及基质轻微水肿,炎症细胞浸润减少; 盐酸左氧氟沙星眼用即型凝胶组角膜无水肿,炎症细胞浸润减少,基质完整。各组兔眼角膜病理显微镜观察见图 2。
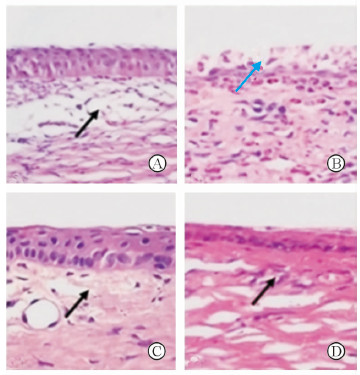
|
图 2 兔角膜病理显微镜观察 Fig 2 Histopathology of rabbit cornea |
2.3 角膜菌落计数
接种菌24 h基线组兔眼角膜菌落计数为(6.83±0.65) CFU。第7天时,盐酸左氧氟沙星滴眼液组和盐酸左氧氟沙星眼用即型凝胶组兔眼角膜菌落计数大幅度减少,分别为(4.87±0.05) CFU和(4.64±0.10) CFU,相比于24 h基线组和PBS空白对照组[(5.65±0.09) CFU]差异均有统计学意义(P=0.00),而盐酸左氧氟沙星眼用即型凝胶组角膜菌落计数低于盐酸左氧氟沙星滴眼液组,差异有统计学意义(P=0.00)。
3 讨论盐酸左氧氟沙星为第3代喹诺酮类广谱抗生素,是氧氟沙星的左旋活性体,具有水溶性高、活性强、不良反应低以及临床疗效高等特点[17],用于治疗敏感细菌引起的细菌性结膜炎、细菌性角膜炎、眼睑炎及泪囊炎等,是临床广泛应用的眼科抗菌药物[18]。
临床常用的盐酸左氧氟沙星药物剂型为滴眼液,其生物利用度低,想要在眼表或治疗部位维持一定的药物治疗浓度必须频繁给药,但频繁高浓度给药可能造成不良反应和眼表细胞损伤[19-20],这是治疗失败的一个重要原因。为使药物到达病变部位并达到一定的治疗浓度,必须增加药物在眼表的存留时间[21]。自20世纪60年代开始,国内外研究者致力于眼部给药缓释系统的研究,目的为使药物持续、控释释放以改善眼部药物生物利用度[22-25],如滴眼液中加入黏性赋型剂、制作眼用药物缓释膜等。目前研究热点主要集中在药物凝胶、胶粒系统的研究,其中凝胶剂的研究越来越受到重视[26-27]。Gupta等[28]应用壳聚糖和海藻酸钠制成左氧氟沙星眼用即型凝胶,体外释药测定结果显示该凝胶2 h时释药约34.18%,6.0 h时释药约76.68%,8.0 h时释药约84.08%,释放药物平缓,具有良好的缓释特性。但国内外对新剂型的研究只停留在药物释放阶段,尚未进一步行药效学的研究。
本研究使用以卡波姆和羟丙甲基纤维素作为主要凝胶基质、盐酸左氧氟沙星为模型药物制作盐酸左氧氟沙星眼用即型凝胶,该种剂型国内外尚无研究。卡波姆为丙烯酸键合烯丙基蔗糖或季戊四醇烯丙醚的高分子聚合物[29-30],含有大量羧基,在pH值小于4的酸性条件下羧基几乎不解离,在水中分散溶胀,表现出较低的黏性但不溶解。随pH值的升高,卡波姆羧基解离,负电荷之间的排斥作用致分子链膨胀及伸展,低浓度的卡波姆则形成澄清溶液,而在较高浓度时溶液黏度急剧增加,溶液发生相变转化成凝胶状态[26],分子链间缠结而形成具有一定弹性和强度的半透明凝胶。当pH值位于6~12之间时,卡波姆形成的凝胶表现出最大的黏度[31],可以提高在眼表的存留时间。但若处方中仅用卡波姆作为主要基质,使用量大,溶液呈酸性,使用时会造成眼部刺激[32],因此需相对减少卡波姆的比例。羟丙甲基纤维素又名羟丙甲纤维素或纤维素羟丙甲基醚,也常被用来制作凝胶,该眼用凝胶在处方中加入了羟丙甲基纤维素作为增稠剂[33]。此外,还加入了磷酸氢二钠、磷酸二氢钠、氯化钠、尼泊金乙酯,加入中和剂氢氧化钠调节溶液pH至6.0,以减少对眼部的刺激性。
本研究以盐酸左氧氟沙星滴眼液为对照药物,对盐酸左氧氟沙星眼用即型凝胶的有效性及优效性进行了考察。实验结果证实盐酸左氧氟沙星眼用即型凝胶治疗实验性金黄色葡萄球菌角膜炎较临床常用的盐酸左氧氟沙星滴眼液更能延缓病情的进一步发展,更显著减少角膜细菌菌落数量,改善临床症状。盐酸左氧氟沙星眼用即型凝胶之所以能提高药物生物利用度是因其滴入结膜囊内,在眼部环境下,液态凝胶发生相转变为半固体状态的凝胶,通过增加药物黏度延长与眼表的接触时间并使药液到达鼻黏膜的速度减慢,从而增加药物眼部吸收量,提高患者治疗的顺应性,有益于提高治疗效果。实验结果表明它是一种有效的新型眼用制剂,具有临床使用潜力。
| [1] | DANDAGI P M, BELEKAR A M, MASTIHOLIMATH V S, GADAD A P, SONTAKE V W, SALIAN P S. An improvement of the efficacy of moxifloxacin HCl for the treatment of bacterial keratitis by the formulation of ocular mucoadhesive microspheres[J]. Sci Pharm , 2013, 81 :259–280. DOI:10.3797/(ISSN)0036-8709 |
| [2] | LIU J L, ZHANG W J, LI X D, YANG N, PAN W S, KONG J, et al. Sustained-release genistein from nanostructured lipid carrier suppresses human lens epithelial cell growth[J]. Int J Ophthalmol , 2016, 9 :643–649. |
| [3] | KIM J, SCHLESINGER E B, DESAI T A. Nanostructured materials for ocular delivery:nanodesign for enhanced bioadhesion, transepithelial permeability and sustained delivery[J]. Ther Deliv , 2015, 6 :1365–1376. DOI:10.4155/tde.15.75 |
| [4] | CHAN W, AKHBANBETOVA A, QUANTOCK A J, HEARD C M. Topical delivery of a Rho-kinase inhibitor to the cornea via mucoadhesive film[J]. Eur J Pharm Sci, 2016 May 16.[Epub ahead of print] https://www.researchgate.net/publication/303293355_Topical_delivery_of_a_Rho-kinase_inhibitor_to_the_cornea_via_Mucoadhesive_film |
| [5] | SHAFAIE S, HUTTER V, COOK M T, BROWN M B, CHAU D Y S. In vitro cell models for ophthalmic drug development applications[J]. Biores Open Access , 2016, 5 :94–108. DOI:10.1089/biores.2016.0008 |
| [6] | SHARMA A K, ARYA A, SAHOO P K, MAJUMDAR D K. Overview of biopolymers as carriers of antiphlogistic agents for treatment of diverse ocular inflammations[J]. Mater Sci Eng C Mater Biol Appl , 2016, 67 :779–791. DOI:10.1016/j.msec.2016.05.060 |
| [7] | BABA K, TANAKA Y, KUBOTA A, KASAI H, YOKOKURA S, NAKANISHI H, et al. A method for enhancing the ocular penetration of eye drops using nanoparticles of hydrolysable dye[J]. J Contr Rel , 2011, 153 :278–287. DOI:10.1016/j.jconrel.2011.04.019 |
| [8] | LI X, ZHANG Z, LI J, SUN S, WENG Y, CHEN H. Diclofenac/biodegradable polymer micelles for ocular applications[J]. Nanoscale , 2012, 4 :4667–4673. DOI:10.1039/c2nr30924f |
| [9] | 张治强, 张玮, 陈光, 张敏, 霍俊滨, 崔磊, 等. pH敏感型盐酸左氧氟沙星眼用即型凝胶的制备及其体外释放考察[J]. 药学实践杂志 , 2010, 28 :122–125. |
| [10] | 高静, 储藏, 丁雪鹰, 张淑瑜, 刘长海, 高申. 盐酸左氧氟沙星眼用即形凝胶在兔眼房水内的药动学[J]. 中国药学杂志 , 2007, 42 :1723–1725. |
| [11] | SANDERS M E, MOORE Q C 3rd, NORCROSS E W, SHAFIEE A, MARQUART M E. Efficacy of besifloxacin in an early treatment model of methicillin-resistant Staphylococcus aureus keratitis[J]. J Ocul Pharmacol Ther , 2010, 26 :193–198. DOI:10.1089/jop.2009.0121 |
| [12] | NORCROSS E W, SANDERS M E, MOORE Q C 3rd, TAYLOR S D, TULLOS N A, CASTON R R, et al. Active immunization with pneumolysinversus 23-valent polysaccharide vaccine for Streptococcus pneumoniae keratitis[J]. Invest Ophthalmol Vis Sci , 2011, 52 :9232–9243. DOI:10.1167/iovs.10-6968 |
| [13] | CHEN K, WU Y, ZHU M, DENG Q, NIE X, LI M, et al. Lithium chloride promotes host resistance against Pseudomonas aeruginosa keratitis[J]. Mol Vis , 2013, 19 :1502–1514. |
| [14] | BERGER E A, MCCLELLAN S A, VISTISEN K S, HAZLETT L D. HIF-1α is essential for effective pmn bacterial killing, antimicrobial peptide production and apoptosis in Pseudomonas aeruginosa keratitis[J]. PLoS Pathog , 2013, 9 :e1003457. DOI:10.1371/journal.ppat.1003457 |
| [15] | SHEN F H, WANG S W, YEH T M, TUNG Y Y, HSU S M, CHEN S H. Absence of CXCL10 aggravates herpes stromal keratitis with reduced primary neutrophil influx in mice[J]. J Virol , 2013, 87 :8502–8510. DOI:10.1128/JVI.01198-13 |
| [16] | MIYABE M, JUNQUEIRA J C, COSTA A C, JORGE A O, RIBEIRO M S, FEIST I S. Effect of photodynamic therapy on clinical isolates of Staphylococcus spp[J]. Braz Oral Res , 2011, 25 :230–234. DOI:10.1590/S1806-83242011005000006 |
| [17] | SONG M, ANG T L. Second and third line treatment options for Helicobacter pylori eradication[J]. World J Gastroenterol , 2014, 20 :1517–1528. DOI:10.3748/wjg.v20.i6.1517 |
| [18] | WATANABE R, NAKAZAWA T, YOKOKURA S, KUBOTA A, KUBOTA H, NISHIDA K. Fluoroquinolone antibacterial eye drops:effects on normal human corneal epithelium, stroma, and endothelium[J]. Clin Ophthalmol , 2010, 4 :1181–1187. |
| [19] | CHOLKAR K, GILGER B C, MITRA A K. Topical, aqueous, clear cyclosporine formulation design for anterior and posterior ocular delivery[J]. Transl Vis Sci Technol , 2015, 4 :1. |
| [20] | MAHMOUD A A, EL-FEKY G S, KAMEL R, AWAD G E. Chitosan/sulfobutylether-β-cyclodextrinnan oparticlesas a potential approach forocular drug delivery[J]. Int J Pharm , 2011, 413 (1-2) :229–236. DOI:10.1016/j.ijpharm.2011.04.031 |
| [21] | HWANG Y S, CHIANG P R, HONG W H, CHIAO C C, CHU I M, HSIUE G H, et al. Study in vivo intraocular biocompatibility of in situ gelation hydrogels:poly(2-ethyl oxazoline)-block-poly(ε-caprolactone)-block-poly(2-ethyl oxazoline) copolymer, matrigel and pluronic F127[J]. PLoS One , 2013, 8 :e67495. DOI:10.1371/journal.pone.0067495 |
| [22] | ZHU X, SU M, TANG S, WANG L, LIANG X, MENG F, et al. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery[J]. Mol Vis , 2012, 18 :1973–1982. |
| [23] | ASASUTJARIT R, THANASANCHOKPIBULL S, FUONGFUCHAT A, VEERANONDHA S. Optimization and evaluation of thermoresponsive diclofenac sodium ophthalmic in situ gels[J]. Int J Pharm , 2011, 411 (1/2) :128–135. |
| [24] | GUPTA H, AQIL M, KHAR R K, ALI A, BHATNAGAR A, MITTAL G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery[J]. J Drug Target , 2011, 19 :409–417. DOI:10.3109/1061186X.2010.504268 |
| [25] | CASOLARO M, CASOLARO I, LAMPONI S. Stimuli-responsive hydrogels for controlled pilocarpine ocular delivery[J]. Eur J Pharm Biopharm , 2012, 80 :553–561. DOI:10.1016/j.ejpb.2011.11.013 |
| [26] | KUMAR D, JAIN N, GULATI N, NAGAICH U. Nanoparticles laden in situ gelling system for ocular drug targeting[J]. J Adv Pharm Technol Res , 2013, 4 :9–17. DOI:10.4103/2231-4040.107495 |
| [27] | BAUMANN M D, KANG C E, TATOR C H, SHOICHET M S. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury[J]. Biomaterials , 2010, 31 :7631–7639. DOI:10.1016/j.biomaterials.2010.07.004 |
| [28] | GUPTA H, AQIL M, KHAR R K, ALI A, BHATNAGAR A, MITTAL G. An alternative in situ gel-formulation of levofloxacin eye drops for prolong ocular retention[J]. J Pharm Bioallied Sci , 2015, 7 :9–14. |
| [29] | BOTSALI M S, TOKAY U, OZMEN B, CORTCU M, KOYUTURK A E, KAHVECIOGLU F. Effect of new innovative restorative carbomised glass cement on intrapulpal temperature rise:an ex-vivo study[J]. Braz Oral Res , 2016, 30 . DOI:10.1590/1807-3107BOR-2016.vol30.0067 |
| [30] | CINTRA G A, PINTO L A, CALIXTO G M, SOARES C P, VON ZUBEN EDE S, SCARPA M V. Bioadhesive surfactant systems for methotrexate skin delivery[J]. Molecules , 2016, 21 . DOI:10.3390/molecules21020231 |
| [31] | JAMBANINJ D, SULAIMAN S A, GILLANI S W, DAVAASUREN T S, ERDENETSETSEG G, DUNGERDORJ D. Technological study of preparing gel from semi-solid extract of Cacalia hastata L[J]. J Adv Pharm Technol Res , 2012, 3 :25–29. |
| [32] | ZOU J, DU L, LI M, LIU B, ZHU W, JIN Y. Transdermal enhancement effect and mechanism of iontophoresis for ono-steroidal anti-inflammatory drugs[J]. Int J Pharm , 2014, 466 (1/2) :76–82. |
| [33] | GHARTI K, THAPA P, BUDHATHOKI U, BHARGAVA A. Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug[J]. J Young Pharm , 2012, 4 :201–208. DOI:10.4103/0975-1483.104363 |
 2016, Vol. 37
2016, Vol. 37


