β-淀粉样蛋白(β-amyloid peptides, Aβ)是老年斑的主要成分,具有广泛的细胞毒作用。Aβ可调节抗凋亡及促凋亡蛋白的表达,引起细胞凋亡[1];产生或诱导大量自由基,加剧氧化应激[2];分泌促炎因子,介导炎症损伤[3]。近期研究发现自噬也参与了Aβ的细胞毒作用[4]。当自噬溶酶体功能障碍时,Aβ降解减少,导致Aβ在脑内异常聚集;Aβ的异常聚集又进一步加重自噬溶酶体功能障碍,形成恶性循环[5]。硫化氢(hydrogen sulfide,H2S)是一种气体信号分子,可以通过调节多种信号途径对Aβ诱导的细胞毒性起到保护作用[6-8]。然而,H2S对Aβ诱导的自噬是否具有保护作用还未见报道。本实验拟通过使用Aβ25-35及H2S供体硫氢化钠(NaHS)干预鼠成神经细胞瘤细胞Neuro-2a,观察其对细胞存活率、自噬蛋白表达及磷脂酰肌醇3-激酶(PI3K)/蛋白激酶B(Akt)/哺乳动物雷帕霉素靶蛋白(mTOR)信号通路的影响,从而探讨H2S对Aβ25-35诱导的Neuro-2a细胞自噬的影响及其潜在机制。
1 材料和方法 1.1 主要试剂和仪器MEM、DMEM高糖培养基、10%澳洲胎牛血清及0.5%青霉素、链霉素购自Gibco公司,Aβ25-35购自生工生物工程(上海)股份有限公司。NaHS、LY294002、3-甲基腺嘌呤(3-MA)及抗LC3B抗体购自Sigma公司,抗Beclin-1、P62抗体购自Abcam公司,抗p-Akt、p-mTOR、GAPDH抗体购自CST公司。MTT细胞毒性检测试剂盒购自碧云天生物技术研究所。辣根过氧化酶标记的二抗及荧光二抗购自武汉博士德生物公司,其余均为国产分析纯试剂。3111型恒温CO2培养箱购自美国Thermo公司,IX71型倒置相差显微镜购自日本Olympus公司,Axio ObserverZI型荧光显微镜购自德国Carl Zeiss公司,Hitachi-7500透射电镜购自日本Hitachi公司。
1.2 细胞培养、分组及药物处理鼠神经瘤母细胞株Neuro-2a购自中国科学院上海生命科学研究院细胞资源中心。细胞培养于含10%胎牛血清的MEM+DMEM高糖培养基中,在37℃、5% CO2培养箱中培养,每2 d更换1次培养基,取对数生长期细胞用于实验。细胞被分为空白对照组、20μmol/L Aβ25-35处理组(Aβ25-35组)、20μmol/L Aβ25-35+100μmol/L NaHS处理组(Aβ25-35+ NaHS组)、20μmol/L Aβ25-35+5μmol/L 3-MA处理组(Aβ25-35+ 3-MA组)、100μmol/L NaHS处理组(NaHS组)及20μmol/L Aβ25-35+100μmol/L NaHS+20μmol/L LY294002处理组(Aβ25-35+ NaHS+ LY294002组)。3-MA及NaHS预处理2 h后加用Aβ25-35继续处理24 h,LY294002在Aβ25-35处理前0.5 h加入。Aβ25-35在使用前置于37℃孵箱中孵育7 d,以获得凝聚态Aβ25-35。Aβ25-35及NaHS的合适工作浓度通过参考文献[8]及前期预实验确定。
1.3 MTT法检测细胞存活率取对数生长期的细胞,以5×103/孔的密度种植至96孔细胞培养板中,贴壁分化后各组分别加入各试剂孵育,24 h后每孔加入10μL的MTT试剂,37℃避光孵育4 h后加入100μL DMSO,15 min后于ELx800多功能酶标仪(BioTek)上测定光密度值,设定波长为560 nm。扣除空白孔中本底的光密度值后,以各处理组与空白对照组的比值表示相对细胞存活率。独立实验重复3次。
1.4 蛋白质印迹法检测相关蛋白表达取对数生长期的细胞,加入蛋白提取裂解液和蛋白酶抑制剂后,在冰上放置30 min,然后4℃条件下14 000×g离心10 min。BCA法测定蛋白浓度,取等量总蛋白与5×SDS上样缓冲液混合,95℃变性5 min,行SDS-PAGE后将蛋白转移至PVDF膜上,5%脱脂奶粉封闭1~2 h,膜孵育一抗(1∶1 000)4℃过夜。TBST洗涤3次,37℃孵育相应二抗(1∶1 000)2 h,于VILBER FUSION FX7 Spectra凝胶成像分析系统上检测并分析蛋白条带。
1.5 免疫荧光检测LC3表达细胞接种于96孔板,经药物处理24 h后,4%多聚甲醛固定共培养的细胞,PBS冲洗,5%山羊血清封闭30 min,加入抗LC3抗体后4℃孵育过夜。PBS冲洗后,加入荧光二抗,37℃避光孵育2 h,DAPI染色后荧光显微镜下观察。
1.6 透射电镜法观察自噬体的形成细胞经药物处理后,用胰酶消化并收集细胞,于4℃离心5 min,弃上清,将标本经2.5%戊二醛固定过夜,加入1%四氧化锇后再次固定2 h。乙醇逐级脱水,环氧树脂包埋。应用切片机切成超薄切片,1%水化醋酸铀和枸橼酸铅染色。于Hitachi-7500透射电镜下观察细胞内自噬体的形成及分布。
1.7 统计学处理所有数据采用SPSS 17.0软件进行统计分析,数据以±s表示,组间比较采用LSD法。检验水准(α)为0.05。
2 结果 2.1 NaHS对Aβ25-35处理后细胞存活率的影响细胞活力检测结果(图 1)显示,与空白对照组相比,Aβ25-35组细胞存活率降低(P < 0.05);而经自噬抑制剂3-MA处理后,细胞存活率增加(P < 0.05),说明自噬可能是Aβ25-35引起细胞损伤的原因之一。NaHS组细胞存活率与空白对照组相比未见明显差异,而Aβ25-35+NaHS组细胞存活率与Aβ25-35组相比升高(P < 0.05)。表明100μmol/L NaHS无细胞毒作用,且可以改善Aβ25-35引起的细胞损伤。
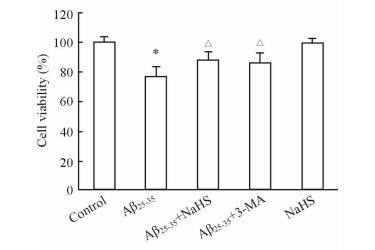
|
图 1 MTT法检测Neuro-2a细胞活性 Fig 1 Viability of Neuro-2a cells was detected by MTT assay in each group |
2.2 NaHS对Aβ25-35引起的自噬的影响
20μmol/L Aβ25-35作用细胞24 h后,Beclin-1的表达及LC3-Ⅱ/ LC3-Ⅰ比值均高于空白对照组,P62表达低于空白对照组(P < 0.05);加入3-MA后Beclin-1及LC3-Ⅱ的表达被抑制,表明Aβ25-35能诱导自噬发生;而NaHS组与正常对照组相比无明显改变。与Aβ25-35组相比,Aβ25-35+ NaHS组Beclin-1表达及LC3-Ⅱ/LC3-Ⅰ比值降低,P62表达增高(P < 0.05, 图 2)。上述结果表明外源性H2S可以降低Aβ25-35引起的自噬活性,但其对自噬的抑制强度弱于3-MA。
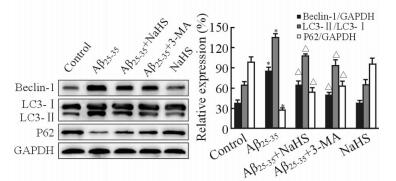
|
图 2 蛋白质印迹法检测Beclin-1、LC3及P62的表达 Fig 2 Expression of Beclin-1, LC3 and P62 proteinwas determined by Western blotting analysis in each group |
2.3 细胞免疫荧光观察LC3的表达和分布
用荧光标记的抗LC3抗体染色后,在荧光显微镜下观察代表自噬体的荧光斑点,结果在空白对照组细胞质内可观察到散在斑点状结构(图 3A);Aβ25-35作用细胞后,胞质内斑点状结构明显增多,且簇集融合呈斑块状(图 3B),表明自噬体增多;3-MA抑制自噬后自噬斑点明显减少(图 3C)。而经NaHS干预后,胞质中荧光斑点较Aβ25-35组减少(图 3D),说明外源性H2S能抑制Aβ25-35引起的自噬活化。
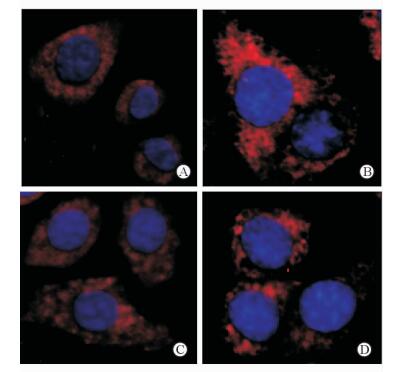
|
图 3 免疫荧光法观察细胞内LC3的表达和分布 Fig 3 Expression and distribution of LC3 by immunofluorence in cells of each group |
2.4 透射电镜下观察自噬体的变化
透射电镜下空白对照组细胞中可见细胞核、线粒体等正常细胞器形态,未见自噬体(图 4A)。Aβ25-35组细胞内可见特征性的双层膜结构自噬体,内含待降解细胞器成分(图 4B);而Aβ25-35+3-MA组及Aβ25-35+NaHS组细胞内自噬体数量较Aβ25-35组明显减少(图 4C、4D)。
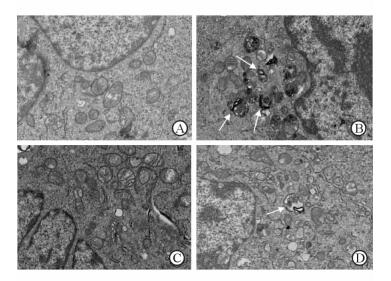
|
图 4 透射电镜下观察自噬体的形成 Fig 4 Autophagosome formation under transmission electron microscope |
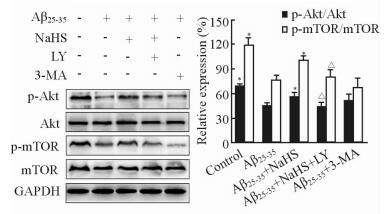
2.5 NaHS对PI3K/Akt/mTOR通路的影响
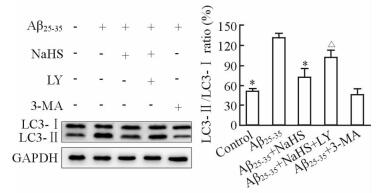
蛋白质印迹结果表明,与空白对照组相比,Aβ25-35组细胞中p-Akt/Akt及p-mTOR/mTOR比值降低,p-Akt及p-mTOR的表达减少(P < 0.05);而Aβ25-35+ NaHS组细胞p-Akt/Akt及p-mTOR/mTOR比值较Aβ25-35组均升高(P < 0.05),但仍未恢复到正常水平(图 5)。当Aβ25-35、NaHS与PI3K/Akt通路特异抑制剂LY294002共孵育细胞时,NaHS对p-Akt及p-mTOR的活化作用被LY294002抑制(P < 0.05,图 5);而NaHS对LC3-Ⅱ表达的降低作用也受到LY294002抑制(P < 0.05,图 6)。上述结果提示外源性H2S可能通过活化PI3K/Akt/mTOR通路来抑制Aβ25-35引起的自噬。
|
图 5 蛋白质印迹法检测p-Akt及p-mTOR的表达 Fig 5 Expression of p-Akt and p-mTOR was measured by Western blotting analysis in each group |
|
图 6 蛋白质印迹法检测LC3的表达 Fig 6 Expression of LC3 was measured by Western blotting analysis in each group |
3 讨论
Aβ25-35是Aβ-40和Aβ1-42的核心片段,具有片段小、易合成、毒性强、更容易透过细胞膜等特点[9],是研究Aβ毒性作用的常用片段。既往研究通过免疫染色法发现,Aβ及其产生途径中的关键酶和肽段存在于神经元自噬溶酶体中,提示自噬体可能是Aβ产生的场所[10]。此外,在早期阿尔茨海默病患者的脑组织中发现Beclin-1蛋白的表达是降低的,在体外培养的神经元和小鼠在体实验中,过表达或沉默Beclin-1也可引起Aβ表达水平的改变[11]。这些研究表明,自噬与Aβ的病理作用密切相关。
本实验结果显示,Aβ25-35降低了细胞存活率,同时诱导自噬蛋白表达增加;而自噬抑制剂3-MA能降低自噬活性,并改善细胞活力,说明Aβ25-35诱导的自噬介导了细胞损伤。有研究者通过向正常小鼠海马中注入Aβ25-35,2周后通过透射电镜发现大量堆积的自噬囊泡,表明毒性Aβ蛋白可以过度活化自噬[12-13],而自噬活性的改变可以损伤溶酶体中底物的降解,引起溶酶体膜通透性增加,组织蛋白酶释放;也可损伤线粒体自噬引起坏死线粒体堆积,线粒体膜通透性增加,最终致神经细胞死亡[14]。
H2S具有重要的生物活性,在体内主要以H2S气体形式和NaHS形式存在,并形成动态平衡。而NaHS溶液较气体H2S更容易确定浓度,方便实验操作,常作为H2S的供体应用于实验[15]。体内外研究证实, H2S对细胞凋亡[16]、炎性损伤[17-18]、氧化应激[8]等均有调节作用。在一项心肌缺血再灌注损伤的动物模型研究中,研究者发现H2S可以通过重建自噬流保护心肌缺血再灌注损伤[19]。Zhang等[20]研究发现H2S可以抑制自噬活性从而对创伤后的脑损伤起到保护作用,表明H2S可能对自噬具有调节作用。自噬是将细胞内长寿命蛋白、受损细胞器等物质转运到溶酶体进行降解并重新利用的生物过程,它区别于蛋白酶体,后者只能降解蛋白质[21]。自噬发生时,Beclin-1、LC3及P62蛋白分别参与了自噬的起始、延长、降解等阶段。其中,LC3是自噬活性的标志蛋白,胞质型LC3-Ⅰ经酶解转化为膜型LC3-Ⅱ,其转化比值可反映自噬水平。P62是一种受体蛋白,介导细胞选择性自噬,其表达水平与自噬活性呈负相关[22]。我们前期实验发现,100μmol/L的外源性H2S对细胞保护作用最为明显,这与既往研究报道哺乳动物脑内H2S的生理浓度约为50~160μmol/L相一致[23]。本实验用100μmol/L NaHS预处理细胞后,Beclin-1表达及LC3-Ⅱ/LC3-Ⅰ比值降低,P62表达增高,说明Aβ25-35引起的自噬活化被抑制。
既往实验发现,Aβ25-35可以下调PI3K/Akt/mTOR信号通路[24],而H2S可以通过活化PI3K/Akt通路减少Aβ的产生[25]。PI3K/Akt被认为是细胞内最重要的抗凋亡、促存活的信号转导通路。PI3K家族包括PI3K-Ⅰ、-Ⅱ和-Ⅲ,其中PI3K-Ⅰ和PI3K-Ⅲ分别参与了抑制和促进自噬[26];mTOR是自噬调控的关键蛋白,其活化后可以抑制自噬起始分子的功能,从而负性调节自噬的活性;Akt是PI3K的下游效应分子,它的激活可促使mTOR活化,可能是联系促细胞生存信号通路与自噬抑制通路的关键蛋白[27]。Aβ25-35导致PI3K/Akt/mTOR通路失活,从而启动自噬过程,改变细胞内的各种能量代谢过程[11]。本研究显示外源性H2S能具有上调p-Akt和p-mTOR的表达,改善Aβ25-35对PI3K/Akt/mTOR通路的抑制作用,而PI3K/Akt/mTOR特异抑制剂LY294002可以部分地阻断外源性H2S对该通路及自噬活性的调节作用,表明外源性H2S抑制Aβ25-35引起的自噬性死亡可能与活化PI3K/Akt/mTOR信号转导通路有关。近期一项针对心肌纤维化的动物实验也证实了H2S具有上调PI3K/Akt通路抑制自噬的作用[28]。
本实验探讨了外源性H2S对Aβ25-35诱导的自噬性细胞死亡的影响,结果发现Aβ25-35下调PI3K/Akt/mTOR信号通路,导致细胞损伤;而外源性H2S通过上调PI3K/Akt/mTOR细胞信号通路使自噬保持低水平状态,从而具有细胞保护作用。下一步研究将通过调节内源性H2S表达及在体实验进一步验证上述结果。
| [1] | IVINS K J, BUI E T, COTMAN C W. β-Amyloid induces local neurite degeneration in cultured hippocampal neurons:evidence for neuritic apoptosis[J]. Neurobiol Dis , 1998, 5 :365–378. DOI:10.1006/nbdi.1998.0228 |
| [2] | BUTTERFIELD D A, SWOMLEY A M, SULTANA R. Amyloidβ-peptide (1-42)-induced oxidative stress in Alzheimer disease:importance in disease pathogenesis and progression[J]. Antioxid Redox Signal , 2013, 19 :823–835. DOI:10.1089/ars.2012.5027 |
| [3] | MCGEER P L, MCGEER E G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease:implications for therapy[J]. Acta Neuropathol , 2013, 126 :479–497. DOI:10.1007/s00401-013-1177-7 |
| [4] | ZHENG L, TERMAN A, HALLBECK M, DEHVARI N, COWBURN R F, BENEDIKZ E, et al. Macroautophagy-generated increase of lysosomal amyloidβ-protein mediates oxidant-induced apoptosis of cultured neuroblastoma cells[J]. Autophagy , 2011, 7 :1528–1545. DOI:10.4161/auto.7.12.18051 |
| [5] | PEREZ S E, HE B, NADEEM M, WUU J, GINSBERG S D, IKONOMOVIC M D, et al. Hippocampal endosomal, lysosomal, and autophagic dysregulation in mild cognitive impairment:correlation with Aβand tau pathology[J]. J Neuropathol Exp Neurol , 2015, 74 :345–358. DOI:10.1097/NEN.0000000000000179 |
| [6] | WEI H J, LI X, TANG X Q. Therapeutic benefits of H2S in Alzheimer's disease[J]. J Clin Neurosci , 2014, 21 :1665–1669. DOI:10.1016/j.jocn.2014.01.006 |
| [7] | LIU Y Y, BIAN J S. Hydrogen sulfide protects amyloid-βinduced cell toxicity in microglia[J]. J Alzheimers Dis , 2010, 22 :1189–1200. |
| [8] | TANG X Q, YANG C T, CHEN J, YIN W L, TIAN S W, HU B, et al. Effect of hydrogen sulphide onβ-amyloid-induced damage in PC12 cells[J]. Clin Exp Pharmacol Physiol , 2008, 35 :180–186. |
| [9] | VARADARAJAN S, KANSKI J, AKSENOVA M, LAUDERBACK C, BUTTERFIELD D A. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer's Aβ1-42 and Aβ25-35[J]. J Am Chem Soc , 2001, 123 :5625–5631. DOI:10.1021/ja010452r |
| [10] | YU W H, CUERVO A M, KUMAR A, PETERHOFF C M, SCHMIDT S D, LEE J H, et al. Macroautophagy——a novelβ-amyloid peptide-generating pathway activated in Alzheimer's disease[J]. J Cell Biol , 2005, 171 :87–98. DOI:10.1083/jcb.200505082 |
| [11] | PICKFORD F, MASLIAH E, BRITSCHGI M, LUCIN K, NARASIMHAN R, JAEGER P A, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloidβaccumulation in mice[J]. J Clin Invest , 2008, 118 :2190–2199. |
| [12] | FAN S, ZHANG B, LUAN P, GU B, WAN Q, HUANG X, et al. PI3K/AKT/mTOR/p70S6K pathway is involved in Aβ25-35-induced autophagy[J]. Biomed Res Int , 2015, 2015 :161020. |
| [13] | XUE Z, GUO Y, ZHANG S, HUANG L, HE Y, FANG R, et al. β-Asarone attenuates amyloidβ-induced autophagy via Akt/mTOR pathway in PC12 cells[J]. Eur J Pharmacol , 2014, 741 :195–204. DOI:10.1016/j.ejphar.2014.08.006 |
| [14] | GHAVAMI S, SHOJAEI S, YEGANEH B, ANDE S R, JANGAMREDDY J R, MEHRPOUR M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders[J]. Prog Neurobiol , 2014, 112 :24–49. DOI:10.1016/j.pneurobio.2013.10.004 |
| [15] | ABE K, KIMURA H. The possible role of hydrogen sulfide as an endogenous neuromodulator[J]. J Neurosci , 1996, 16 :1066–1071. |
| [16] | YIN W L, HE J Q, HU B, JIANG Z S, TANG X Q. Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells[J]. Life Sci , 2009, 85 :269–275. DOI:10.1016/j.lfs.2009.05.023 |
| [17] | LIU H, DENG Y, GAO J, LIU Y, LI W, SHI J, et al. Sodium hydrosulfide attenuatesβ-amyloid-induced cognitive deficits and neuroinflammation via modulation of MAPK/NF-κB pathway in rats[J]. Curr Alzheimer Res , 2015, 12 :673–683. DOI:10.2174/1567205012666150713102326 |
| [18] | FAN H, GUO Y, LIANG X, YUAN Y, QI X, WANG M, et al. Hydrogen sulfide protects against amyloidβ-peptide induced neuronal injury via attenuating inflammatory responses in a rat model[J]. J Biomed Res , 2013, 27 :296–304. DOI:10.7555/JBR.27.20120100 |
| [19] | XIE H, XU Q, JIA J, AO G, SUN Y, HU L, et al. Hydrogen sulfide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagic flux[J]. Biochem Biophys Res Commun , 2015, 458 :632–638. DOI:10.1016/j.bbrc.2015.02.017 |
| [20] | ZHANG M, SHAN H, CHANG P, WANG T, DONG W, CHEN X, et al. Hydrogen sulfide offers neuroprotection on traumatic brain injury in parallel with reduced apoptosis and autophagy in mice[J]. PLoS One , 2014, 9 . |
| [21] | KROEMER G, LEVINE B. Autophagic cell death:the story of a misnomer[J]. Nat Rev Mol Cell Biol , 2008, 9 :1004–1010. DOI:10.1038/nrm2529 |
| [22] | ESTEBAN-MARTINEZ L, BOYA P. Autophagic flux determination in vivo and ex vivo[J]. Methods , 2015, 75 :79–86. DOI:10.1016/j.ymeth.2015.01.008 |
| [23] | HOSOKI R, MATSUKI N, KIMURA H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide[J]. Biochem Biophys Res Commun , 1997, 237 :527–531. DOI:10.1006/bbrc.1997.6878 |
| [24] | JIN Y, FAN Y, YAN E Z, YANG J, ZONG Z H, QI Z M. Amyliodβ-protein fragment 25-35 down-regulates PI3K/Akt/p70S6K pathway in rat hippocampus in vivo[J]. Zhongguo Yaolixue Yu Dulixue Zazhi , 2007, 21 :90–98. |
| [25] | 陈筱山, 何选丽, 朱丽娟, 张华, 冯飞, 晏勇. 外源性硫化氢对原代神经元早老素1和β淀粉样蛋白的影响[J]. 第三军医大学学报 , 2013, 35 :2060–2064. |
| [26] | HERAS-SANDOVAL D, PÉREZ-ROJAS J M, HERNÁNDEZ-DAMIÁN J, PEDRAZA-CHAVERRI J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration[J]. Cell Signal , 2014, 26 :2694–2701. DOI:10.1016/j.cellsig.2014.08.019 |
| [27] | RODON J, DIENSTMANN R, SERRA V, TABERNERO J. Development of PI3K inhibitors:lessons learned from early clinical trials[J]. Nat Rev Clin Oncol , 2013, 10 :143–153. DOI:10.1038/nrclinonc.2013.10 |
| [28] | XIAO T, LUO J, WU Z, LI F, ZENG O, YANG J. Effects of hydrogen sulfide on myocardial fibrosis and PI3K/AKT1-regulated autophagy in diabetic rats[J]. Mol Med Rep , 2016, 13 :1765–1773. |
 2016, Vol. 37
2016, Vol. 37


