2. 首都医科大学附属北京安贞医院心脏外科, 北京 100029
2. Department of Cardiovascular Surgery, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China
心房颤动(房颤)是临床上最为常见的持续性心律失常,是心血管专科最常见的住院原因之一,房颤不仅严重影响患者的生活质量,而且增加了其远期病死率[1]。国外研究表明,普通人群中约有0.5%的人罹患房颤,房颤患者男性多于女性,且随年龄增长其发病率增高[2-4]。我国房颤发病率为0.77%,标准化后的患病率为0.61%,房颤最重要、危害最大的并发症之一是脑卒中[5]。胡大一等[6]对中国房颤住院病例多中心对照研究结果显示,住院房颤患者脑卒中的发生率达24.81%,且有随年龄增长而增加的趋势,80岁以上脑卒中患病率高达32.86%。房颤的治疗主要有药物治疗、导管消融和外科治疗,3种疗法临床远期随访的结果均不十分满意。深层次揭示房颤发生的机制,对指导房颤的临床治疗、提高人民健康水平有重大意义。
目前认为房颤的发生和维持需要3个条件:触发因素、发生和传导的基质及自主神经系统[7-8]。Li等[9]最先在心衰的研究中描述了弥散性心房纤维化和传导异常在房颤中的重要性,之后的一些临床和亚临床研究[10-19]证实了这一点。近年来各种基础和临床研究提示自主神经系统可以触发房颤[20-22]。心脏脂肪垫(fat pad,FP)位于心外膜的脂肪结缔组织内,其内包含由自主神经下行支配心脏的心脏神经丛,因此近年来对于FP与房颤关系的研究成为热点。此前的研究主要涉及自主神经系统对于阵发性房颤启动的影响[20-22],以及FP消融对阵发性房颤终止的影响[23-24],目前还没有FP在持续性房颤中作用的相关报道。阵发性房颤与持续性房颤及永久性房颤有很大不同,目前迷走神经参与持续性房颤的机制并未阐明,仍有一些问题亟需解决。例如:对于持续性房颤来说,神经丛刺激能否对心房各个部位电生理产生类似于阵发性房颤时的效果,FP激活主要对心房哪些部位产生影响,消融后的电生理影响又如何。
本实验通过心外膜标测的方法研究了持续性房颤模型犬房颤持续时分别刺激右前FP、下腔静脉-心房下部FP和左房背侧FP对同侧肺静脉、肺静脉前庭及心房顶房颤周长的影响;另外还研究了消融下腔静脉-心房下部FP后,刺激右前FP、左房背侧FP对同侧肺静脉、肺静脉前庭及心房顶房颤周长的影响;并比较了FP刺激前、FP刺激时以及下腔静脉-心房下部FP消融后再刺激其他FP时上述部位房颤周长的变化程度。
1 材料和方法 1.1 动物、材料和仪器健康杂种犬22只[北京大学第一医院实验动物中心,许可证号:SYXK(京)2014-0010],随机分为2组:A组12只,利用埋藏式高频率心脏起搏器心外膜快速起搏(600次/min)右心耳,持续起搏8周,建立持续性房颤犬模型;B组10只,为假手术对照。高频电刀(GD350-P,上海沪通电子有限公司); 6F 20极和10极环状电极(Lasso,Biosense Webster);6F 6极和4极标测电极(Biosense Webster);头端直径为 4 mm的大头消融导管(IBI);单道心电图机(FX-2111,北京福田电子医疗仪器有限公司);多通道记录仪(Cathlab computer,GE inc.,Wisconsin,USA);程序刺激仪(Bloom DTU 215B,20 mA,Bloom Electrophysiology Fischer Imaging Corporation,USA);神经刺激仪(动物实验专用,四川锦江电子科技有限公司);射频消融仪(IBI 1500T Cardiac Ablation Generator,Irvine-Biomedical)。
1.2 心外膜标测A组12只持续性房颤犬以戊巴比妥钠(30 mg/kg)静脉注射麻醉,气管内插管,调节呼吸机为容量控制模式,流量4~6 L/min,潮气量为10 mL/kg,呼吸压力0~2 kPa。吸入3%恩氟烷维持麻醉,持续监测心电、血压及动脉血氧饱和度。双胸背部备皮,将用磁铁控制脉冲发生器,停止脉冲发放。
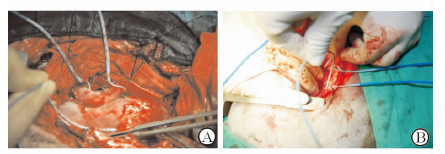
右侧入路(图 1A):犬左侧卧位,常规消毒铺单,右侧第3肋间切口,逐层开胸,湿纱垫推开右肺暴露心脏,纵向剪开心包,悬吊心包。游离右侧肺静脉,采用4极、6极、10极Lasso和20极Lasso电极作为心外膜标测电极,10极Lasso电极(极间距2 mm)环绕右上肺静脉,20极Lasso电极(极间距2 mm)环绕右下肺静脉,4极电极置于右房顶部(1、2极朝向左心房,3、4极在右心房;极间距2 mm),6极电极垂直于肺静脉放于右肺静脉前庭上(1、2极朝向下腔静脉;5、6极位于上腔静脉)。采用大头电极作为刺激电极,将刺激电极连接在程序刺激仪及多导电生理记录仪上,多通道记录仪(滤波0.05~500 Hz)显示和记录体表心电图及心外膜双极电图信号。
|
图 1 右侧入路(A)及左侧入路(B)标测电极位置 Fig 1 Location of mapping electrode through right (A) and left (B) approaches A: Ring electrode located in the right superior pulmonary vein, right inferior pulmonary vein, right pulmonary vein atrium and atrial roof; B: Ring electrode located in the left superior pulmonary vein, left inferior pulmonary vein, left pulmonary vein atrium and atrial roof |
左侧入路(图 1B):右侧电生理检查结束后,将犬改为右侧卧位,常规消毒铺单,左侧第4肋间切口,逐层开胸,湿纱垫推开左肺暴露心脏,左膈神经后纵向剪开心包,悬吊心包。游离左侧肺静脉,从左上和左下肺静脉之间向心房后壁分离,暴露左侧脂肪垫,10极Lasso电极(极间距2 mm)环绕左上肺静脉,20极Lasso电极(极间距2 mm)环绕左下肺静脉,4极电极置于左房顶部(1、2极朝向右心房;3、4极在左心房;极间距2 mm),6极电极垂直于肺静脉放于左肺静脉前庭上(1、2极朝向尾端,5、6极朝向头端),多通道记录仪(滤波0.05~500 Hz)显示和记录体表心电图及心外膜双极电图信号。
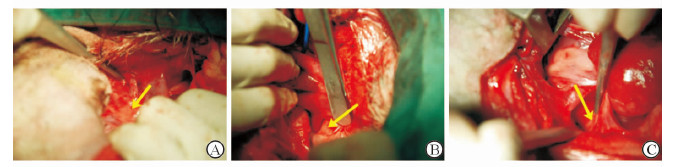
1.3 FP刺激后电生理检查右侧入路:可见右侧两个FP,右前FP位于心房腹侧窦房结尾端邻近右上肺静脉心房连接处(图 2A),下腔静脉-心房下部FP位于下腔静脉和两个心房连接处(图 2B)。大头刺激电极右心耳爆发起搏,S1-S1刺激,周长100 ms,2倍舒张期阈值,持续30 s,诱发持续性房颤。记录右上肺静脉、右下肺静脉、右肺静脉前庭、右房顶、左房顶各部位房颤周长。大头刺激电极连接神经刺激仪行高频刺激(刺激频率20 Hz,双向方波,脉宽0.1 ms,峰值电压2~10 V),分别刺激右房前FP、下腔静脉-心房下部FP,两次刺激间隔5 min。记录上述各标测部位房颤周长。房颤周长定义为心外膜电图 30个连续周期的平均值。心电图激动间隔<100 ms或者持续的心电活动记录为1个间隔。
|
图 2 右前脂肪垫(A)、下腔静脉-心房下部脂肪垫(B)和左房背侧脂肪垫(C) Fig 2 Right anterior fat pad (A), inferior vena cava-inferior atrial fat pad (B) and left atrial dorsal fat pad (C) |
左侧入路:沿左上和左下肺静脉之间钝性分离,在左心房背侧可见左房背侧FP(图 2C)。大头刺激电极左心耳爆发起搏,S1-S1刺激,周长100 ms,2倍舒张期阈值,持续30 s,诱发持续性房颤。记录左上肺静脉、左下肺静脉、左肺静脉前庭、左房顶、右房顶各部位房颤周长。大头刺激电极连接神经刺激仪行高频刺激(刺激频率20 Hz,双向方波,脉宽0.1 ms,峰值电压2~10 V),刺激左房背侧FP,记录上述各标测部位房颤周长。
1.4 下腔静脉-心房下部FP消融后电生理检查犬左侧卧位,经原右侧入路切口按前文方法重新放置右侧标测电极。大头电极连接射频消融仪,消融参数设置为功率30 W,温度43℃,放电时盐水流速17 mL/min,每点放电30~60 s,消融下腔静脉-心房下部FP。大头刺激电极右心耳爆发起搏,S1-S1刺激,周长100 ms,2倍舒张期阈值,持续30 s,诱发持续性房颤。诱发房颤后记录右上肺静脉、右下肺静脉、右肺静脉前庭、右房顶、左房顶各部位房颤周长。重复刺激右前FP,记录右上肺静脉、右下肺静脉、右肺静脉前庭、右房顶、左房顶各部位房颤周长。
1.5 组织学检查A组和B组共22只犬,待A组犬全部电生理研究完成后,处死A组和B组犬,取出心脏,切取右前FP、下腔静脉-心房下部FP和左房背部FP,FP包括神经节和心肌全部切除作为标本。组织置于10%中性甲醛固定,乙醇脱水,二甲苯透明,常规石蜡包埋,切片厚4 μ m,行H-E染色。Olympus BX40型显微镜下观察。所有图像由显微镜分析软件Motic 3.2获得。
1.6 统计学处理采用SPSS 12.0统计学软件分析数据。计量资料均采用x±s表示。刺激前和刺激后的房颤周长比较采用配对t检验。检验水准(α)为0.05。
2 结 果 2.1 FP刺激后电生理检查结果应用爆发刺激方法A组全部12只犬均可诱发持续性房颤。由表 1可见,刺激右前FP后,右上肺静脉、右下肺静脉和右肺静脉前庭房颤周长与刺激前相比缩短(P<0.05),分别缩短 (12.0±2.8)、(10.8±3.9)和(7.9±2.3) ms;右房顶和左房顶房颤周长与刺激前相比差异无统计学意义。刺激下腔静脉-心房下部FP时,右上肺静脉、右下肺静脉和右肺静脉前庭房颤周长与刺激前相比缩短(P<0.05),分别缩短(3.2±1.8)、(11.7±4.2)和(9.2±1.6) ms,其中右下肺静脉周长缩短幅度大于右上肺静脉;右房顶与左房顶房颤周长与刺激前相比差异无统计学意义。左侧入路房颤持续时,刺激左房背侧FP后,左上肺静脉、左下肺静脉和左肺静脉前庭房颤周长与刺激前相比缩短(P<0.05),分别比刺激前缩短(10.0±3.2)、(9.9±2.7)和(9.7±2.3) ms;左房顶与右房顶房颤周长与刺激前相比差异无统计学意义。
|
|
表 1 刺激3种脂肪垫前后标测部位房颤周长变化 Tab 1 Changes of atrial fibrillation cycle length before and after stimulation of the 3 epicardial fat pads |
2.2 下腔静脉-心房下部FP消融后电生理检查结果
下腔静脉-心房下部FP消融后,应用爆发刺激方法A组全部12只犬均可诱发持续性房颤。由表 2可见,消融下腔静脉-心房下部FP后再刺激右前FP,右上肺静脉和右肺静脉前庭房颤周长与刺激前相比缩短(P<0.05),右房顶与左房顶房颤周长与刺激前相比差异无统计学意义;与下腔静脉-心房下部FP未消融不同的是,刺激右前FP后右下肺静脉房颤周长与刺激前相比差异无统计学意义。
|
|
表 2 消融下腔静脉-心房下部脂肪垫后刺激右前脂肪垫前后标测部位房颤周长变化 Tab 2 Changes of atrial fibrillation cycle length before and after stimulation of the right anterior fat pad after ablating inferior vena cava-inferior atrial fat pad |
2.3 组织学检查
在术中全部22只犬均可看到3个FP:右前FP、下腔静脉-心房下部FP、左房背侧FP。FP呈三角型或椭圆形,右前FP位于右房右侧与左房交界处腹侧面;下腔静脉-心房下部FP位于右房背侧与左房相交处邻近下腔静脉起始部;左房背侧FP位于左房背侧靠近左侧肺静脉端。FP消融后,可见局部脂肪坏死,组织形成焦痂。H-E染色显示(图 3):FP由心外膜覆盖,内可见大量脂肪组织,脂肪组织内可见神经节和神经纤维,神经节内可见大量神经细胞胞体。神经纤维走行不一,周围可见滋养血管。FP消融后可见脂肪细胞大量坏死,神经节内神经元胞体大量坏死、空泡化,神经纤维排列散乱,可见纤维素坏死。

|
图 3 犬心外膜脂肪垫组织学检查(H-E染色) Fig 3 Histological examination of epicardial fat pads of dogs (H-E staining) A: Left atrial dorsal fat pad showing rich nerve fibers and nutrient vessels; B: Left atrial dorsal fat pad showing the neurons; C: The ganglion cells and nerve fibers degenerated after ablating the inferior vena cava-inferior atrial fat pad. Original magnification: ×40 (A,C), ×100 (B) |
3 讨 论
近年来,各种基础和临床研究提示自主神经系统可以触发房颤。1994年Coumel[20]提出自主神经张力的改变可以诱发阵发性房颤。Jackman实验室在动物实验中发现,刺激犬肺静脉旁FP内的神经丛可以增加肺静脉内心肌细胞的期前收缩数量,易化实验犬房颤的诱发[21]。Oh等[22]在动物实验中发现当刺激一侧颈部迷走神经时右心耳、右房游离壁、左心耳、左房游离壁房颤可诱导性增加,而进行FP消融后这些迷走神经效应减少。这些研究提示副交感神经系统与阵发性房颤密切相关,迷走神经兴奋时,兴奋可通过心外膜FP来影响心房的电生理属性,可以触发肺静脉内期前收缩从而触发阵发性房颤。本研究通过持续性房颤犬模型评价了在房颤持续时心外膜FP对房颤的影响,研究发现右前FP、下腔静脉-心房下部FP、左房背侧FP刺激可以缩短同侧肺静脉和肺静脉前庭的房颤周长,而对房顶无影响。房颤周长与激动频率相关,激动频率越快,房颤周长越短。房颤周长对于判断主导频率位点、评价手术效果非常重要。有研究显示消融主导频率位点可以延长房颤周长,终止房颤[23-24]。本研究提示心外膜FP对于持续性房颤仍存在影响,FP兴奋可以通过选择性地引起肺静脉和肺静脉前庭的快速激动来维持房颤持续。
另外,先前的研究还提示不同FP对于不同部位心房的电生理影响不同。Hou等[25]研究发现,犬右前神经丛刺激可以引起房室间期延长,心室率和窦性节律减慢;而消融右下神经丛后,窦性节律减慢效应不变而房室传导抑制消失。左上神经丛刺激可以引起窦房结和房室结类似的功能效应,而当消融右下神经丛后窦率减慢效应减少。这项研究提示,各个FP间不是孤立的,是相互协同的。本研究发现,刺激下腔静脉-心房下部FP时,右下肺静脉房颤周长缩短幅度大于右上肺静脉[(11.7±4.2) ms vs (3.2±1.8) ms],消融下腔静脉-心房下部FP后,刺激右前FP引起右下肺静脉房颤周长缩短的效果消失[(96.6±3.1) ms vs (96.4±3.0) ms],这提示右前FP和下腔静脉-心房下部FP对于右侧肺静脉的影响不同,但又存在协同效应。右前FP主要影响右上肺静脉并通过下腔静脉-心房下部FP来影响右下肺静脉,而下腔静脉-心房下部FP对于右下肺静脉的影响要大于右上肺静脉。
在临床方面,Pappone等[26]对297例房颤患者行环肺静脉消融过程中发现,34%的患者左房内某些位点对高能量射频电流产生心动过缓等迷走反应,针对这些位点继续消融可使迷走反应消失。经过12个月的随访,99%术中有迷走反应的患者无房颤复发,而术中没有迷走反应的患者仅有74%无房颤复发。Scherlag等[27]比较了常规消融治疗+FP消融(联合治疗组)与单纯常规消融治疗(常规治疗组)的房颤患者的疗效,通过对联合治疗组平均5个月和常规治疗组平均12个月的随访发现,联合治疗组91%的患者无房颤发作,包括17例阵发性房颤(100%,17/17)和13例持续性房颤(81%,13/16);常规治疗组无房颤发作者仅70%(19/27),包括10例阵发性房颤(71%,10/14)和9例持续性房颤(69%,9/13)。McClelland等[28]应用微创手术在行肺静脉前庭隔离时附加神经丛消融治疗房颤,全组房颤患者20例,对于16例阵发和持续性房颤患者成功率达87.5%。Greif等[29]认为,增厚的心外膜FP与房颤及左房重构(左房扩张)密切相关。有文献报道心外膜FP可生成激活素A,后者引起心房纤维化,可能导致房颤[30]。Mazurek等[31]通过PET检查发现,房颤组心外膜FP的炎性活动高于对照组。Iacobellis等[32]通过超声心动图检查心外膜FP发现,永久性房颤患者的FP厚度高于阵发性房颤者。Nakahara等[33]通过临床随访发现左心房合并FP消融是简便可行的持续性房颤消融策略。
与以往研究不同,本研究以持续性房颤模型为研究对象,并采用多导联、多部位心外膜标测的方法来探讨心外膜FP参与持续性房颤维持的作用机制。本研究有2个主要发现:(1) 在持续性房颤时,犬3个主要FP(右前FP、下腔静脉-心房下部FP、左房背侧FP)接受刺激后可以缩短同侧肺静脉及肺静脉前庭的房颤周长。上述部位快速激动伴颤动样传导维持房颤持续。(2) 右前FP与下腔静脉-心房下部FP影响范围有所不同。下腔静脉-心房下部FP接受刺激后主要引起右下肺静脉和右肺静脉前庭房颤周长缩短。右前FP接受刺激后可以引起右上、右下肺静脉及右肺静脉前庭的房颤周长缩短,但其引起右下肺静脉的房颤周长变化是通过下腔静脉-心房下部FP实现,右前FP与下腔静脉-心房下部FP存在联系。
本研究提示,在持续性房颤中副交感神经系统对于房颤的维持起重要作用,可以作为持续性房颤消融治疗的重要靶点。3个主要FP主要通过影响肺静脉及前庭的房颤周长来起作用,充分的肺静脉隔离可以减弱迷走神经对持续性房颤的维持效应。由于标测仪器的技术限制,我们只能记录有限导联的心外膜电图,因此在本研究中只记录了FP对同侧肺静脉、肺静脉前庭和有限心房的心外膜电图,而未记录对侧区域,这可能局限了我们对FP影响范围的认识。同样由于技术的限制我们把FP相互影响的研究集中在位于同侧的右前FP和下腔静脉-心房下部FP。
| [1] | Wong C X, Brooks A G, Leong D P, Roberts-Thomson K C, Sanders P. The increasing burden of atrial fibrillation compared with heart failure and myocardial infarction:a 15-year study of all hospitalizations in Australia. Arch Intern Med[J]. 2012, 172 :739–741 . |
| [2] | Prystowsky E N, Benson D W Jr, Fuster V, Hart R G, Kay G N, Myerburg R J, et al. Management of patients with atrial fibrillation:a statement for healthcare professionals from the subcommittee on electrocardiography and electrophysiology, American Heart Association. Circulation[J]. 1996, 93 :1262–1277 . |
| [3] | Brand F N, Abbott R D, Kannel W B, Wolf P A. Characteristics and prognosis of lone atrial fibrillation:30-year follow-up in the Framingham Study. JAMA[J]. 1985, 254 :3449–3453 . |
| [4] | Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation as an independent risk factor for stroke:the Framingham Study. Stroke[J]. 1991, 22 :983–988 . |
| [5] | 周自强, 胡大一, 陈捷, 张仁汉, 李奎宝, 赵秀丽. 中国心房颤动现状的流行病学研究. 中华内科杂志[J]. 2004,43 :491–494. |
| [6] | 胡大一, 孙艺红, 周自强, 李奎宝, 倪永斌, 杨光, 等. 中国人非瓣膜性心房颤动脑卒中危险因素的病例对照研究. 中华内科杂志[J]. 2003,42 :157–161. |
| [7] | Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation:a translational appraisal. Physiol Rev[J]. 2011, 91 :265–325 . |
| [8] | Hatem S N, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res[J]. 2014, 102 :205–213 . |
| [9] | Li D, Fareh S, Leung T K, Nattel S. Promotion of atrial fibrillation by heart failure in dogs:atrial remodeling of a different sort. Circulation[J]. 1999, 100 :87–95 . |
| [10] | Sanders P, Morton J B, Davidson N C, Spence S J, Vohra J K, Sparks P B, et al. Electrical remodeling of the atria in congestive heart failure:electrophysiological and electroanatomic mapping in humans. Circulation[J]. 2003, 108 :1461–1468 . |
| [11] | Lau D H, Mackenzie L, Kelly D J, Psaltis P J, Worthington M, Rajendram A, et al. Short-term hypertension is associated with the development of atrial fibrillation substrate:a study in an ovine hypertensive model. Heart Rhythm[J]. 2010, 7 :396–404 . |
| [12] | Medi C, Kalman J M, Spence S J, Teh A W, Lee G, Bader I, et al. Atrial electrical and structural changes associated with longstanding hypertension in humans:implications for the substrate for atrial fibrillation. J Cardiovasc Electrophysiol[J]. 2011, 22 :1317–1324 . |
| [13] | Alasady M, Shipp N J, Brooks A G, Lim H S, Lau D H, Barlow D, et al. Myocardial infarction and atrial fibrillation:importance of atrial ischemia. Circ Arrhythm Electrophysiol[J]. 2013, 6 :738–745 . |
| [14] | Sanders P, Morton J B, Kistler P M, Spence S J, Davidson N C, Hussin A, et al. Electrophysiological and electroanatomic characterization of the atria in sinus node disease:evidence of diffuse atrial remodeling. Circulation[J]. 2004, 109 :1514–1522 . |
| [15] | John B, Stiles M K, Kuklik P, Brooks A G, Chandy S T, Kalman J M, et al. Reverse remodeling of the atria after treatment of chronic stretch in humans:implications for the atrial fibrillation substrate. J Am Coll Cardiol[J]. 2010, 55 :1217–1226 . |
| [16] | Roberts-Thomson K C, John B, Worthley S G, Brooks A G, Stiles M K, Lau D H, et al. Left atrial remodeling in patients with atrial septal defects. Heart Rhythm[J]. 2009, 6 :1000–1006 . |
| [17] | Dimitri H, Ng M, Brooks A G, Kuklik P, Stiles M K, Lau D H, et al. Atrial remodeling in obstructive sleep apnea:implications for atrial fibrillation. Heart Rhythm[J]. 2012, 9 :321–327 . |
| [18] | Lau D H, Middeldorp M E, Brooks A G, Ganesan A N, Roberts-Thomson K C, Stiles M K, et al. Aortic stiffness in lone atrial fibrillation:a novel risk factor for arrhythmia recurrence. PLoS One[J]. 2013, 8 :e76776. |
| [19] | Abed H S, Samuel C S, Lau D H, Kelly D J, Royce S G, Alasady M, et al. Obesity results in progressive atrial structural and electrical remodeling:implications for atrial fibrillation. Heart Rhythm[J]. 2013, 10 :90–100 . |
| [20] | Coumel P. Paroxysmal atrial fibrillation:a disorder of autonomic tone. Eur Heart J[J]. 1994, 15 (Suppl A) :9–16 . |
| [21] | Scherlag B J, Yamanashi W, Patel U, Lazzara R, Jackman W M. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol[J]. 2005, 45 :1878–1886 . |
| [22] | Oh S, Zhang Y, Bibevski S, Marrouche N F, Natale A, Mazgalev T N. Vagal denervation and atrial fibrillation inducibility:epicardial fat pad ablation does not have long-term effects. Heart Rhythm[J]. 2006, 3 :701–708 . |
| [23] | Sanders P, Berenfeld O, Hocini M, Jaïs P, Vaidyanathan R, Hsu L F, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation[J]. 2005, 112 :789–797 . |
| [24] | Haïssaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, et al. Catheter ablation of long-lasting persistent atrial fibrillation:critical structures for termination. J Cardiovasc Electrophysiol[J]. 2005, 16 :1125–1137 . |
| [25] | Hou Y, Scherlag B J, Lin J, Zhou J, Song J, Zhang Y, et al. Interactive atrial neural network:determining the connections between ganglionated plexi. Heart Rhythm[J]. 2007, 4 :56–63 . |
| [26] | Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation[J]. 2004, 109 :327–334 . |
| [27] | Scherlag B J, Nakagawa H, Jackman W M, Yamanashi W S, Patterson E, Po S, et al. Electrical stimulation to identify neural elements on the heart:their role in atrial fibrillation. J Interv Card Electrophysiol[J]. 2005, 13 (Suppl 1) :37–42 . |
| [28] | McClelland J H, Duke D, Reddy R. Preliminary results of a limited thoracotomy:new approach to treat atrial fibrillation. J Cardiovasc Electrophysiol[J]. 2007, 18 :1289–1295 . |
| [29] | Greif M, von Ziegler F, Wakili R, Tittus J, Becker C, Helbig S, et al. Increased pericardial adipose tissue is correlated with atrial fibrillation and left atrial dilatation. Clin Res Cardiol[J]. 2013, 102 :555–562 . |
| [30] | Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J[J]. 2015, 36 :795–805a . |
| [31] | Mazurek T, Kiliszek M, Kobylecka M, Skubisz-Głuchowska J, Kochman J, Filipiak K, et al. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am J Cardiol[J]. 2014, 113 :1505–1508 . |
| [32] | Iacobellis G, Zaki M C, Garcia D, Willens H J. Epicardial fat in atrial fibrillation and heart failure. Horm Metab Res[J]. 2014, 46 :587–590 . |
| [33] | Nakahara S, Hori Y, Kobayashi S, Sakai Y, Taguchi I, Takayanagi K, et al. Epicardial adipose tissue-based defragmentation approach to persistent atrial fibrillation:its impact on complex fractionated electrograms and ablation outcome. Heart Rhythm[J]. 2014, 11 :1343–1351 . |
 2016, Vol. 37
2016, Vol. 37


