急性肾损伤 (acute kidney injury, AKI)是指在肾前性、肾性以及肾后性损害因素作用下,肾功能在数天内急剧下降[1,2,3]。 AKI镜下病理通常表现为肾小管上皮细胞坏死脱落、肾小管腔内管型形成和间质炎细胞浸润[4]。动物实验和前瞻性临床研究均发现,经历AKI打击的肾脏即使短期肾功能恢复,长期进展为慢性肾脏病 (chronic kidney disease,CKD)的风险也大于未发生过AKI者[5,6]。
由AKI进展到以肾小管间质纤维化为病理特征的CKD的具体机制尚不明确,可能与AKI发生后局部修复性过程的慢性失衡及其与对抗性因子复杂的相互作用有关。这类修复性进程主要包括细胞周期在损伤后的阻滞、缺氧诱导因子的分泌和转化生长因子β(transforming growth factor β,TGF-β)信号通路的激活等[7 ,8,9]。2012年Kitagawa等[10]发现一种以血小板源性生长因子受体α (PDGFR-α)依赖性的方式调节细胞间质分泌的蛋白——PHD锌指蛋白14(PHF14),并制作了PHF14基因非条件敲除小鼠模型。动物模型证明PHF14非条件敲除具有致死性,小鼠出生后存活不到24 h,死因为严重的肺纤维化造成的呼吸衰竭,尸检提示肝和肾都有明显的纤维化。体外实验证明PHF14可负向调节PDGFR-α的表达,从而抑制成纤维细胞的细胞外基质分泌[10,11]。由此说明PHF14基因在胚胎发育阶段各器官细胞外基质形成平衡中起重要调节作用。
本研究探讨野生型小鼠在遭受AKI打击时PHF14表达变化规律,并通过对PHF14成年期诱导敲除小鼠模型进行AKI打击,考察PHF14在其后续纤维化进程中是否有调节作用。
1 材料和方法 1.1 AKI动物模型的制备及分组IVC级成年C57BL/6野生型小鼠(购自美国JAX实验室), 8~12周龄,体质量20~35 g,饲养于第二军医大学实验动物中心。按随机数字表法将20只动物分为2组: 对照组(n=5)和实验组(n=15)。实验组再分为叶酸作用2、14和28 d 3个亚组,每个亚组5只小鼠。实验组所有动物给予叶酸(溶于0.3 mol/L NaHCO3中,叶酸终浓度为10 g/L)腹腔注射,剂量250 mg/kg,叶酸由大连美仑生物技术有限公司提供。实验组动物分别于腹腔给药后2、14和28 d时取血,处死小鼠,留取肾脏组织。对照组动物给予0.3 mol/L NaHCO3腹腔注射(剂量25 mL/kg)后取血,处死小鼠,留取肾脏组织。
1.2 PHF14成年特异性诱导敲除模型小鼠制作phf14 loxp+/+小鼠[11]由中国科学院上海生命科学研究院生物化学与细胞生物学研究所陈正军教授赠送。他莫昔芬诱导Cre表达小鼠(Cre-ERTM)由美国JAX实验室提供[B6.Cg-Tg(Cre/Esr1)5Amc/J mice (stock 004682)],phf14 lox+/+小鼠与Cre-ERTM杂交,子一代筛选phf14 lox+/-;Cre+小鼠自交,子二代中筛选出phf14 lox+/+;Cre+小鼠饲养至8~12周,每组取8只作为给予肾损伤刺激后不同时间点的实验组研究对象;与每个时间点对应,另取每组同月龄phf14 lox+/+; Cre- 8只作为对照组。实验组与对照组均连续5 d腹腔注射他莫昔芬,剂量3 mg/40 g;溶媒为玉米油(Sigma),他莫昔芬在玉米油中的终浓度为7.5 mg/mL[12]。第6天两组均给予叶酸腹腔注射,剂量250 mg/kg。所有动物正常饲养,第6、8、19和33天时处死小鼠,并留取肾脏组织。
1.3 血肌酐(SCr)及血尿素氮(BUN)检测眼眶取血法取血1 mL于EDTA促凝管中,静置过夜后1 377×g室温离心10 min,取血清至无菌Eppendorf管中,-80℃冻存。全自动生化分析仪测定SCr和BUN。
1.4 蛋白质免疫印迹法检测肾脏PHF14、α-平滑肌肌动蛋白(α-SMA)、collagen 1、TGF-β的表达用组织裂解液(0.1% SDS,0.5%脱氧胆酸钠,150 mmol/L NaCl,50 mmol/L Tris-HCl pH 8.0,0.5% 钒酸钠,1% NP-40,1 mmol/L NaF,蛋白酶抑制剂)裂解组织后4℃离心取上清,BCA法测定蛋白浓度。取20 μg蛋白,8% SDS-PAGE,半干法转至PVDF膜。一抗分别为anti-PHF14(Abcam,1∶1 000),GAPDH(Santa Cruz,1∶500)、α-SMA(Abcam,1∶1 000)、collagen 1(SAB,1∶400),ECL反应,X线胶片曝光,全自动凝胶成像系统进行分析。结果用Image J进行灰度半定量分析。
1.5 肾组织病理学检查肾组织用4%多聚甲醛固定,石蜡包埋,切片,行Masson染色以及α-SMA、collagen 1免疫组织化学染色,显微镜下观察。病理切片和免疫组化染色也用Image J进行半定量分析。
1.6 统计学处理统计学分析采用GraphPad Prism 6.0 进行数据分析。计量资料使用x±s表示。经检验数据为正态分布并且方差齐后使用两独立样本t检验,不符合正态分布或方差齐性的数据则采用Kruskal-Wallis检验。检验水准(α)为0.05。
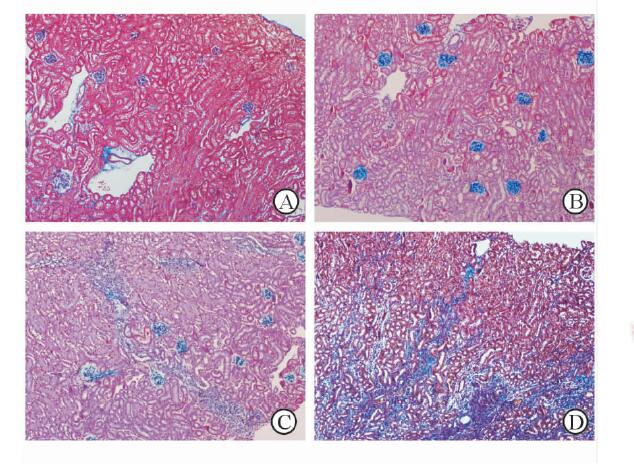
2 结 果 2.1 急性损伤后肾纤维化模型的成功构建如表 1,小鼠在叶酸注射后第2天发生AKI,肾功能明显下降;在第14天,小鼠度过了AKI病程肾功能明显下降的阶段,进入慢性纤维化时期,SCr及BUN水平有所恢复但仍较基线有轻度升高;第28天时SCr及BUN水平已经进一步恢复。不同时间点小鼠SCr及BUN水平均符合AKI病程,病理切片Masson染色和免疫组化可见对照组小鼠肾脏病理表现基本正常(图 1A),而叶酸刺激2 d组小鼠纤维化尚不明显(图 1B)。叶酸给药后14~28 d则呈不断进展的肾间质纤维化,呈多灶状纤维化,伴淋巴细胞和单核细胞浸润,肾小管上皮细胞萎缩、变性,肾小管管腔扩大(图图 1C、图 1D)。以上表明叶酸性肾病模型制作成功。
|
|
表 1 接受叶酸刺激后不同时间点小鼠肾功能检测结果 Tab 1 Renal function of the mice at the indicated time points after folic acid insult |
|
图 1 接受叶酸刺激后小鼠肾脏病理切片(Masson染色) Fig 1 Renal morphologic analyses (Masson) of the mice after folic acid insult Renal Masson’s trichrome for mice after medium injection (A) , day 2 (B), day 14 (C) and day 28 (D) after folic acid injection demonstrated that 250 mg/kg folic administration induced notable renal fibrosis. Original magnification: ×100 |
蛋白质印迹检测结果显示,和对照组相比,叶酸刺激的小鼠从第2天开始肾脏PHF14表达上调,并且在后续纤维化形成过程中持续高表达(图 2),各时间点相对灰度分别为:第0天0.654±0.048、第2天0.837±0.337、第14天1.454±0.061、第28天1.477±0.058,第2、14和28天与第0天相比,差异有统计学意义(P<0.05),提示PHF14的高表达与纤维化相关信号转导通路激活相关。

|
图 2 叶酸刺激后小鼠肾脏PHF14表达变化 Fig 2 Renal PHF14 protein expression profile after folic acid administration at different time points FA: Folic acid; PHF14: Plant homodomain finger protein 14; GAPDH: Reduced glyceraldehyde-phosphate dehydrogenase |
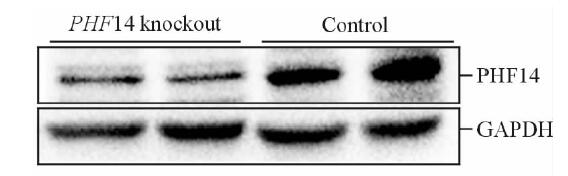
基因诱导敲除后,蛋白质印迹法检测结果显示肾脏PHF14表达下调(图 3),条带相对灰度值PHF14敲除鼠(0.414±0.104)弱于PHF14未敲除组(1.000±0.172),差异有统计学意义(P<0.05)。
|
图 3 他莫昔芬诱导后phf14 lox+/+; Cre+和phf14 lox+/+; Cre-小鼠肾脏PHF14表达量 Fig 3 PHF14 expression level of mice with genotype of phf14 lox+/+, Cre+ and phf14 lox+/+, and Cre- after tamoxifen injection After the five days’ administration of tamoxifen, Western blotting results demonstrated the suppressed PHF14 expression level in the mice of PHF14 knockout group. PHF14: Plant homodomain finger protein 14; GAPDH: Reduced glyceraldehyde-phosphate dehydrogenase |
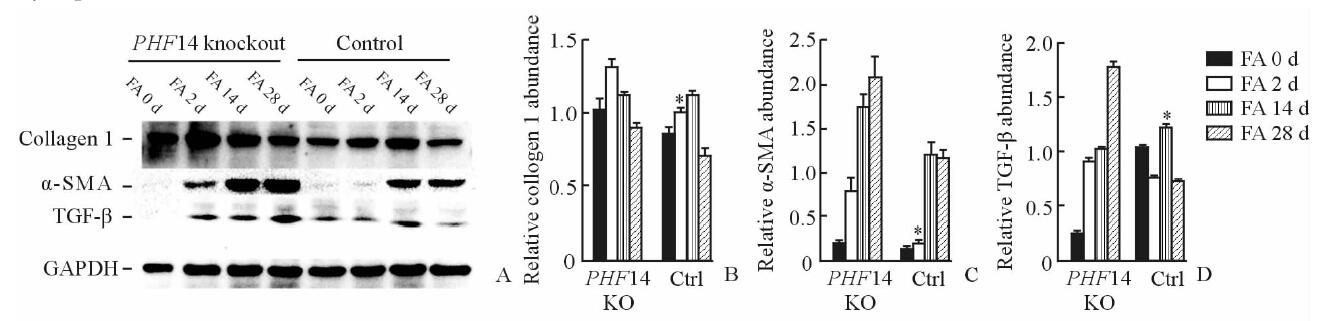
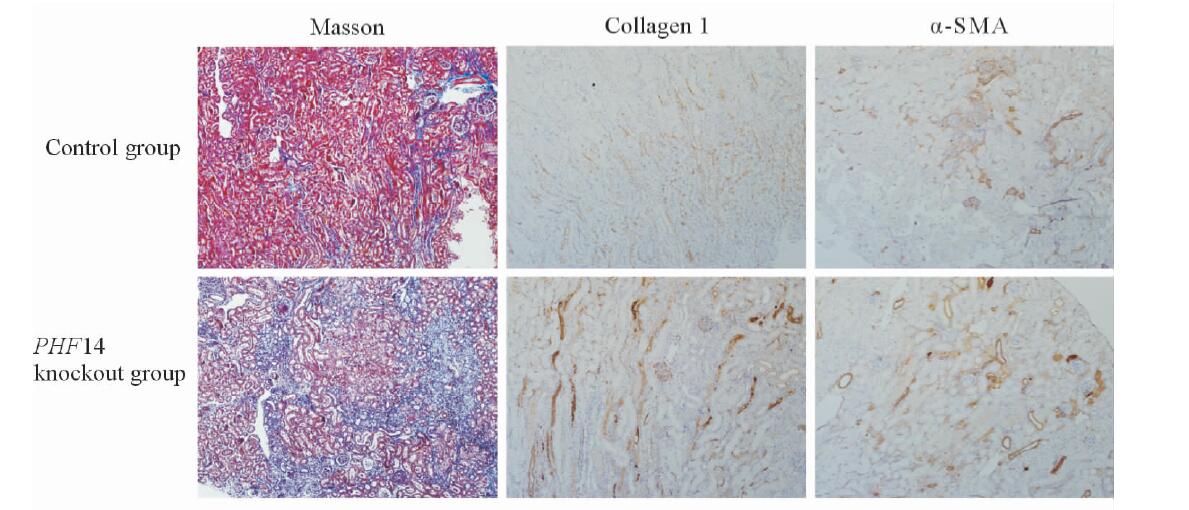
蛋白质印迹法检测结果(图 4A)显示,在腹腔注射叶酸后2、14和28 d时,相较野生型组,PHF14基因敲除鼠的collagen 1表达上升更早,并且表达上升水平也更高(P<0.05,图 4B);α-SMA的变化规律也类似(图 4C);对照组TGF-β的表达水平在给予叶酸后14 d达高峰,在28 d时已经回落,但PHF14敲除鼠在接受叶酸刺激后TGF-β表现出持续的升高,最高值也较未敲除组更高(图 4D)。对照组小鼠和PHF14敲除组小鼠在叶酸刺激28 d时,肾脏Masson染色显示PHF14敲除组纤维化更重,collagen 1及α-SMA表达上调(图 5),这与蛋白质印迹法检测结果一致。
|
图 4 PHF14敲除鼠和对照组小鼠在接受叶酸刺激后肾脏纤维化相关蛋白表达(A)及灰度扫描(B~D)结果 Fig 4 Fibrosis-related proteins expression(A) and gray scanning(B-D) in the PHF14 knockout mice and control mice after folic acid administration PHF14: Plant homodomain finger protein 14; α-SMA:α-smooth muscle actin; TGF-β: Transforming growth factor β; GAPDH: Reduced glyceraldehyde-phosphate dehydrogenase; FA: Folic acid; KO: Knockout; Ctrl: Control. *P<0.05 vs PHF14 knockout group. n=8, x±s |
|
图 5 对照组小鼠与PHF14敲除小鼠在给予叶酸28 d后的肾脏Masson染色切片以及collagen 1、α-SMA免疫组织化学染色 Fig 5 Renal Masson’s trichrome, collagen 1 and α-SMA immunohistochemical staining of the control mice and PHF14 knockout mice at day 28 after folic acid administration Renal Masson’s trichrome showed more severe fibrotic pathological findings in PHF14 knockout mice. Immunohistochemical staining revealed that collagen 1 and α-SMA syntheses were also increased compared with control littermates. PHF14: Plant homodomain finger protein 14; α-SMA: α-smooth muscle actin. Original magnification: ×100 |
肾小管间质纤维化是慢性肾衰竭的病理基础[13,14],探究AKI后肾小管间质纤维化的机制对CKD的防治有重要意义。本研究发现肾脏PHF14基因在叶酸造成的肾损伤模型中发生上调,在该模型的慢性纤维化期持续高表达,并且与纤维化的进展有时间依赖关系,这提示PHF14与纤维化表型相关。PHF14成年期诱导敲除的小鼠在遭到AKI打击后的28 d时表现出较遭到同样打击的野生型小鼠更为严重的肾纤维化。以上结果提示PHF14基因受促纤维化刺激而上调,但其作用是抑制纤维化进程,成年期PHF14基因敲除会造成AKI后纤维化加剧。之前在胚胎期动物关于PHF14基因作用的研究发现,其在胚胎形成阶段各器官间质组织形成中起负向调节作用,并且证明其作用机制是PHF14蛋白结合于PDGFR-α转录起始位点抑制其转录,进而抑制由PDGF信号通路所介导的间质形成[10,11]。PDGF是生长因子家族中的一员,它与其他生长因子信号通路有着复杂的网络相互作用,并且同样受TGF-β调节。PDGF信号通路的激活会导致间质细胞的增殖与细胞外基质合成的增加,并且作用强度较强[15,16]。研究显示,PHF14基因敲除的小鼠胚胎各器官PDGFR-α表达过度,PDGF信号通路过度激活,间质形成过多;在病理水平上表现为纤维化,受累组织包括肝、肺、肾等重要器官;该基因敲除小鼠出生后由于肺纤维化通气功能不足,因呼吸衰竭死亡[10,11]。由于所使用的非条件敲除动物模型的局限性,前人的工作无法阐明成年期该基因在肾脏遭到促纤维化打击时是否发挥作用,本研究结果肯定了这一点。在使用基因修饰动物模型方面,我们通过杂交产生PHF14基因条件敲除模型,这样避免了初生小鼠死亡,从而可以创造条件研究PHF14在成年期小鼠肾脏中的作用。
在损伤后慢性纤维化模型的选择上,本研究采用了叶酸刺激性肾损伤模型。这是肾毒性因素造成急性肾损伤的常用模型,叶酸刺激后肾功能变化完全模拟临床AKI病程,并且造模后纤维化发生比例较高,混杂因素少,动物个体间及造模批次间重复性较好,可以重复监测肾功能[17]。在该模型中我们验证了PHF14对抗纤维化的作用。但在不同的种属、不同的肾脏纤维化模型中,促进纤维化发展的具体机制并不相同,如活化的间质成纤维细胞的主要来源及上皮间质转化是否参与在不同的模型中均有差异[18]。PHF14在不同种属和不同促纤维化刺激模型中所发挥的作用及重要性仍有待验证。
已经有大量研究致力于阐明肾纤维化的机制,尽管该过程涉及多种细胞系和细胞因子的参与,目前普遍认为TGF-β信号通路在成年期肾纤维化中起核心作用,是纤维化的始动和维持因子[19,20,21,22]。成纤维细胞能接受TGF-β刺激而活化为肌成纤维细胞,后者是肾纤维化中细胞外基质的主要来源。在多种AKI模型中均发现,TGF-β能够破坏小管上皮细胞的完整性并促进上皮间质转化。TGF-β对局部的炎症和免疫反应也有复杂的调节作用,并能加剧毛细血管的萎缩消失[23]。鉴于在我们的研究中发现PHF14的表达与肾纤维化进程有相关性,并且可以负向调节细胞外基质的产生,合理的推测是TGF-β通过其信号转导通路激活了PHF14的表达,PHF14蛋白表达增多,抑制PDGFR-α的转录,进而减少成纤维细胞的活化和细胞外基质的分泌,从宏观上看则减轻肾小管间质纤维化的程度。这样的环状机制成为防止TGF-β过度促纤维化的自限性机制,该自限机制缺乏或下调均会促进成年期肾脏在遭到AKI打击后加速进展到纤维化。至于TGF-β以何种方式上调PHF14的转录和表达尚不明确。目前已知在胚胎期PHF14通过抑制PDGFR-α的转录起到抑制纤维化表型的作用,在成年期其作用方式是否相同需要进一步研究验证。如果TGF-β在纤维化进程中通过PHF14形成自限的机制得到阐明,则该自限信号通路上的靶点有望成为新的抗纤维化治疗作用位点。
| [1] | Waikar S S, Liu K D, Chertow G M. Diagnosis, epidemiology and outcomes of acute kidney injury[J]. Clin J Am Soc Nephrol, 2008, 3:844-861. |
| [2] | Mehta R L, Kellum J A, Shah S V, Molitoris B A, Ronco C, Warnock D G, et al. Acute Kidney Injury Network:report of an initiative to improve outcomes in acute kidney injury[J]. Crit Care, 2007, 11:R31. |
| [3] | Chertow G M, Burdick E, Honour M, Bonventre J V, Bates D W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients[J]. J Am Soc Nephrol, 2005, 16:3365-3370. |
| [4] | Chawla L S, Eggers P W, Star R A, Kimmel P L. Acute kidney injury and chronic kidney disease as interconnected syndromes[J]. N Engl J Med, 2014, 371:58-66. |
| [5] | Rifkin D E, Coca S G, Kalantar-Zadeh K. Does AKI truly lead to CKD?[J]. J Am Soc Nephrol, 2012, 23:979-984. |
| [6] | Leung K C, Tonelli M, James M T. Chronic kidney disease following acute kidney injury-risk and outcomes[J]. Nat Rev Nephrol, 2013, 9:77-85. |
| [7] | Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition[J]. Am J Physiol Renal Physiol, 2014, 307:F1187-F1195. |
| [8] | Pannu N. Bidirectional relationships between acute kidney injury and chronic kidney disease[J]. Curr Opin Nephrol Hypertens, 2013, 22:351-356. |
| [9] | García-Sánchez O, López-Hernández F J, López-Novoa J M. An integrative view on the role of TGF-β in the progressive tubular deletion associated with chronic kidney disease[J]. Kidney Int, 2010, 77:950-955. |
| [10] | Kitagawa M, Takebe A, Ono Y, Imai T, Nakao K, Nishikawa S, et al. Phf14, a novel regulator of mesenchyme growth via platelet-derived growth factor (PDGF) receptor-α[J]. J Biol Chem, 2012,287:27983-27996. |
| [11] | Huang Q, Zhang L, Wang Y, Zhang C, Zhou S, Yang G, et al. Depletion of PHF14, a novel histone-binding protein gene, causes neonatal lethality in mice due to respiratory failure[J]. Acta Biochim Biophys Sin, 2013, 45:622-633. |
| [12] | Hayashi S, McMahon A P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre:a tool for temporally regulated gene activation/inactivation in the mouse[J]. Dev Biol, 2002, 244:305-318. |
| [13] | Goldstein S L, Jaber B L, Faubel S, Chawla L S; Acute Kidney Injury Advisory Group of American Society of Nephrology. AKI transition of care:a potential opportunity to detect and prevent CKD[J]. Clin J Am Soc Nephrol, 2013, 8:476-483. |
| [14] | Chawla L S, Kimmel P L. Acute kidney injury and chronic kidney disease:an integrated clinical syndrome[J]. Kidney Int, 2012, 82:516-524. |
| [15] | Nakagawa T, Inoue H, Sasahara M. Platelet-derived growth factor and renal disease[J]. Curr Opin Nephrol Hypertens, 2012, 21:80-85. |
| [16] | Kok H M, Falke L L, Goldschmeding R, Nguyen T Q. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease[J]. Nat Rev Nephrol, 2014, 10:700-711. |
| [17] | Eddy A A, López-Guisa J M, Okamura D M, Yamaguchi I. Investigating mechanisms of chronic kidney disease in mouse models[J]. Pediatr Nephrol, 2012, 27:1233-1247. |
| [18] | Tsutomu I, Akihiro U, Tsuneo T, Hiromichi S, Hirokazu O. The contribution of epithelial-mesenchymal transition to renal fibrosis differs among kidney disease models[J]. Kidney Int, 2014, 87:233-238. |
| [19] | Zeisberg M, Neilson E G. Mechanisms of tubulointerstitial fibrosis[J]. J Am Soc Nephrol, 2010, 21:1819-1834. |
| [20] | Eddy A A. Overview of the cellular and molecular basis of kidney fibrosis[J]. Kidney Int Suppl, 2014, 4:2-8. |
| [21] | Akhurst R J, Hata A. Targeting the TGF-β signalling pathway in disease[J]. Nat Rev Drug Discov, 2012, 11:790-811. |
| [22] | Liu Y. Cellular and molecular mechanisms of renal fibrosis[J]. Nat Rev Nephrol, 2011, 7:684-696. |
| [23] | Gewin L, Zent R. How does TGF-β mediate tubulointerstitial fibrosis?[J]. Semin Nephrol, 2012, 32:228-235. |
 2016, Vol. 37
2016, Vol. 37


