2. 南京军区南京总医院国家肾脏疾病临床医学研究中心, 全军肾脏病研究所, 南京 210016;
3. 解放军97医院徐州医学院附属淮海医院药剂科, 徐州 221004;
4. 徐州医学院感染与免疫实验室, 徐州 221004;
5. 徐州医学院附属医院肾内科, 徐州 221000
2. National Clinical Research Center for Kidney Diseases, PLA Institute of Kidney Disease, General Hospital, PLA Nanjing Military Area Command, Nanjing 210016, Jiangsu, China;
3. Department of Pharmacy, No. 97 Hospital of PLA, Huaihai Hospital Affiliated to Xuzhou Medical College, Xuzhou 221004, Jiangsu, China;
4. Laboratory of Infection and Immunity, Xuzhou Medical College, Xuzhou 221004, Jiangsu, China;
5. Department of Nephrology, Affiliated Hospital of Xuzhou Medical College, Xuzhou 221000, Jiangsu, China
甘西鼠尾草(Salvia przewalskii Maxim.)为唇形科(lamiaceae)鼠尾草属(salvia)植物,又名大紫丹参、甘肃丹参等,文献报道其药理活性主要有抑制超氧自由基及抗氧化[1]、醛糖还原酶抑制活性[2]等。采用大孔吸附树脂分离纯化制备的甘西鼠尾草总酚酸提取物(Salvia przewalskii extract of total phenolic acids,SPE)主要含有迷迭香酸(rosmarinic acid,RA)和丹酚酸B(salvianolic acid B,SalB)等水溶性酚酸类成分[3],能够降低血清病性肾小球肾炎模型大鼠的蛋白尿和正常大鼠的全血黏度[4, 5]。本课题组前期体内实验发现,SPE对嘌呤霉素氨基核苷(puromycin aminonucleoside,PAN)肾病大鼠足细胞损伤有一定的保护作用,并且可以降低PAN肾病大鼠蛋白尿[6]。
至于SPE是通过何种单体成分发挥保护足细胞的作用以及其保护足细胞的具体作用机制,目前尚不明确。既往研究已证实PAN诱导的肾小球足细胞损伤与活性氧(reactive oxygen species,ROS)产生过多有关[7, 8, 9, 10, 11],ROS与其相关的细胞因子相互作用,导致细胞毒性作用发生[12],最严重的氧化应激损伤是DNA损伤,体现在8-羟基脱氧鸟苷(8-hydroxy-2’-deoxyguanine,8-OHdG)的形成[13]。因而,本课题采用PAN肾病大鼠模型和体外PAN损伤足细胞模型,探讨SPE及其单体是否抑制PAN所致足细胞氧化应激损伤,从而具有保护足细胞的作用。
1 材料和方法 1.1 SPE及其单体制备SPE由徐州医学院附属淮海医院药剂科中药化学实验室提供,为甘西鼠尾草干燥根茎药材按照固定工艺[3]制备得到,批号20090901-b15,采用高效液相色谱法进行含量测定(面积归一化法),测得该提取物中RA含量为31.58%、SalB含量为5.52%[5]。使用前加入1%羧甲基纤维素钠(CMC-Na)水溶液配制成 5、10和 20 mg/mL的溶液,现配现用。RA对照品、SalB对照品由本课题组自制[14],经高效液相色谱检查(面积归一化法)纯度均大于99%。
1.2 主要试剂PAN(Sigma,美国),他克莫司(安斯泰来),3,3-二氨基联苯胺(DAB,北京中杉金桥生物技术有限公司),8-OHdG(Trevigrn,美国),蛋白酶K(Sigma,美国),RNase A(Sigma,美国),Alexa Fluor 488标记山羊抗兔IgG(上海碧云天生物技术有限公司),兔抗WT1、羊抗兔(Santa Cruz,美国),胎牛血清(ExCell Biology),RPMI1640培养基、青霉素和链霉素溶液、0.05%Trypsin-EDTA(Gibco,美国),鬼笔环肽(Santa Cruz,美国),ROS检测试剂盒(上海碧云天生物技术有限公司)。
1.3 实验动物雄性清洁级SD大鼠,体质量150~180 g[徐州医学院实验动物中心提供,许可证号SCXK(苏)2010-0003],饲养温度控制在24~26℃之间,湿度65%,12 h交替照明。饲养期间给予大鼠普通标准饲料,自由饮食,保持垫料干燥。
1.4 动物模型建立大鼠随机分为6组,正常组、模型对照组、阳性对照(他克莫司)组、SPE低、中、高剂量组,每组各12只。正常组给予生理盐水 80 mg/kg一次性尾静脉注射,其余给予同等剂量PAN尾静脉一次性注射。注射1 d后,每日固定时间正常组、模型对照组以10 mL/kg剂量灌胃给予1% CMC-Na溶液,阳性对照组以2 mg/kg剂量灌胃给予他克莫司溶液,SPE低、中、高剂量组以10 mL/kg剂量分别灌胃给予5、10、20 mg/mL SPE溶液。
1.5 标本留取各组分别在第5、10、15、21天各处死大鼠3只。大鼠腹腔内注射水合氯醛(3.5 mg/kg)麻醉,取腹正中切口,暴露并切取双侧肾脏,肾脏冠状面取组织。肾皮质组织以10%甲醛溶液固定,石蜡包埋,-20℃冰箱保存。
1.6 WT1免疫组化染色石蜡切片常规脱蜡至水,柠檬酸盐缓冲液(pH 6.0)抗原修复,室温自然冷却,PBS洗涤;3% H2O2室温孵育10 min,PBS洗涤;10%山羊血清封闭1 h,滴加兔抗WT1(1:100)4℃孵育过夜。次日室温复温30 min,PBS洗涤,滴加羊抗兔(1:500)37℃孵育30 min,PBS洗涤;DAB染色,苏木精着色2 min,脱水、透明,中性树脂封片后显微镜(Olympus,日本)观察,每个标本取10个肾小球切面,直接在高倍镜下计数WT1阳性细胞数目,取平均值[15]。
1.7 8-OHdG免疫荧光染色2 μm石蜡切片,脱蜡水化,蛋白酶K 37℃孵育20 min,PBS洗涤;浸泡于缓冲液中37℃孵育1 h,PBS洗涤,HCl室温孵育5 min,然后Tris-base浸润5 min,PBS洗涤;10%山羊血清封闭1 h,滴加一抗(1:250)4℃孵育过夜,PBS洗涤,滴加二抗(1:1 000),避光37℃孵育30 min,PBS洗涤;甘油封片,荧光显微镜(Olympus,日本)下观察8-OHdG在各组肾小球中的变化,采用 Image-Pro Plus(IPP)6.0 图像处理分析软件,比较各组荧光强度[16]。
1.8 足细胞培养及分组小鼠条件永生化足细胞系MCP-5由南京军区南京总医院刘志红院士惠赠,本实验室保存。将小鼠足细胞在RPMI 1640培养液[10% 胎牛血清、100 U/mL青霉素及100 mg/L链霉素、10 U/mL干扰素(IFN)-γ],33℃、5%CO2条件下增殖传代。在37℃、5%CO2条件下用无IFN-γ的培养基培养14 d,足细胞停止增殖,获得分化表型[17]。实验分为正常组、模型对照组、阳性对照(他克莫司)组、SPE(高、低剂量)组、SalB(高、低剂量)组、RA(高、低剂量)组。实验前各组细胞用含0.1%胎牛血清的RPMI 1640培养液饥饿过夜,除正常组外各组加入PAN(100 μg/mL)作用24 h,模型对照组给予0.1% DMSO,阳性对照组给予他克莫司1 μg/mL,SPE高、低剂量组分别给予SPE 316、158 μg/mL,SalB高、低剂量组分别给予SalB 17、8.5 μg/mL,RA高、低剂量组分别给予RA 50、25 μg/mL,分别取6、12、24、48 h作为观察点,收集细胞待测。
1.9 足细胞骨架染色取细胞爬片标本,37℃预热PBS洗涤3次,75%冰乙醇固定10 min,PBS洗涤;0.5% Triton X-100通透5 min,PBS洗涤;室温避光条件下鬼笔环肽孵育30 min,PBS洗涤,甘油封片,荧光显微镜(Olympus,日本)观察结果。
1.10 足细胞ROS荧光强度测量上述各组足细胞弃去培养液,37℃预热PBS洗涤,加入稀释后的DCFH-DA工作液(10 μmol/mL),37℃避光孵育30 min,收集细胞,PBS洗涤3次,流式细胞仪(BD FACS Canto Ⅱ)分析各组足细胞内ROS荧光强度,每例样品测定至少10 000个细胞[18]。
1.11 统计学处理用SPSS 16.0软件进行统计学分析。计量资料均以 x±s 表示,两组间样本均数比较采用t检验,多组间比较采用单因素方差分析。检验水准(α)为0.05。
2 结果 2.1 大鼠肾小球足细胞数目的改变第5天时正常组大鼠平均WT1阳性细胞计数为(37.2±1.5)个/肾小球切面,而模型对照组已减少为(23.5±0.9)个/肾小球切面,第15天时模型组达最低值(14.4±0.7)个/肾小球切面,与正常组相比差异均具有统计学意义(P<0.05)。SPE组第15、21天时WT1阳性细胞数量高于模型对照组,其中第15天时高剂量组与阳性对照组保护足细胞效果较为接近[SPE高剂量组(21.7±1.0)个/肾小球切面vs阳性对照组(23.6±1.2)个/肾小球切面,P>0.05]。见图 1。
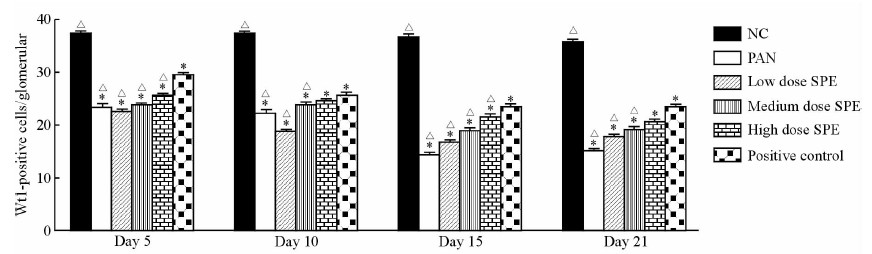
|
图 1 各组大鼠不同时间点肾小球切面WT1阳性细胞计数 Fig 1 Count of WT1-positive cells per glomerular section of rats in each group at different time points vessels NC: Normal control; PAN: Puromycin aminonucleoside (80 mg/kg); Low dose SPE: SPE (5 mg/mL); Medium dose SPE: SPE (10 mg/mL); High dose SPE: SPE (20 mg/mL); Positive control: Tacrolimus (2 mg/kg). SPE: Salvia przewalskii extract of total phenolic acids. *P<0.05 vs NC; △P<0.05 vs positive control. n=3, x±s |
PAN注射后第5天时,模型对照组大鼠肾组织中8-OHdG荧光强度已较正常组升高(P<0.05),第10天时大鼠肾组织中8-OHdG的荧光强度达到最高。给予SPE及他克莫司干预后,大鼠肾组织中8-OHdG的荧光强度低于模型对照组;第15天时阳性对照组、SPE高剂量组大鼠肾组织中8-OHdG的荧光强度明显下降,与正常组相比差异无统计学意义(P>0.05);至第21天各组8-OHdG表达水平均接近正常范围(图 2、3)。

|
图 2 各组大鼠肾组织中各时间点8-OHdG免疫荧光染色结果 Fig 2 Immunofluorescence detection of 8-OHdG in rat renal tissue of each group at different time points vessels NC: Normal control; PAN: Puromycin aminonucleoside (80 mg/kg); Low dose SPE: SPE (5 mg/mL); Medium dose SPE: SPE (10 mg/mL); High dose SPE: SPE (20 mg/mL); Positive control: Tacrolimus (2 mg/kg). SPE: Salvia przewalskii extract of total phenolic acids. Original magnification: ×400 |
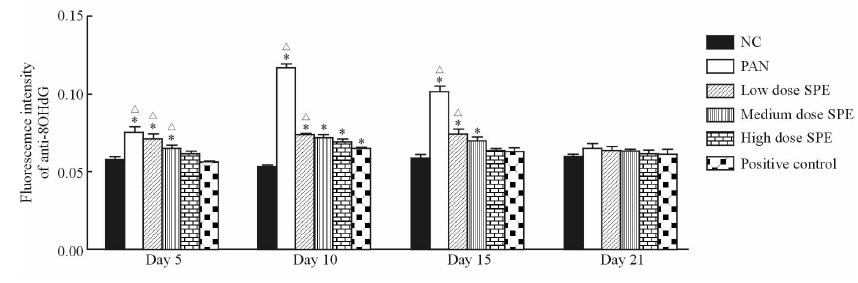
|
图 3 免疫荧光法检测各组大鼠肾组织中各时间点8-OHdG荧光强度的变化 Fig 3 Fluorescence intensities of 8-OHdG in rat renal tissue of each group by immunofluoresence at different time points vessels NC: Normal control; PAN: Puromycin aminonucleoside (80 mg/kg); Low dose SPE: SPE (5 mg/mL); Medium dose SPE: SPE (10 mg/mL); High dose SPE: SPE (20 mg/mL); Positive control: Tacrolimus (2 mg/kg). SPE: Salvia przewalskii extract of total phenolic acids. *P<0.05 vs NC; △P<0.05 vs positive control. n=3, x±s |
正常分化的足细胞F-actin为红色的长条细丝状,高度有序地贯穿于细胞,密度大,丝强劲有力,沿细胞呈极性分布。PAN作用24 h后,F-actin几乎完全解聚,少数细胞尚有残存的被切断的丝状结构;足细胞失去原有的细胞张力,胞体回缩,细胞形态变圆。而给予SPE、SalB、RA及他克莫司干预后,PAN诱导的足细胞损伤明显减弱,细胞内重新出现极性分布的微丝(图 4)。
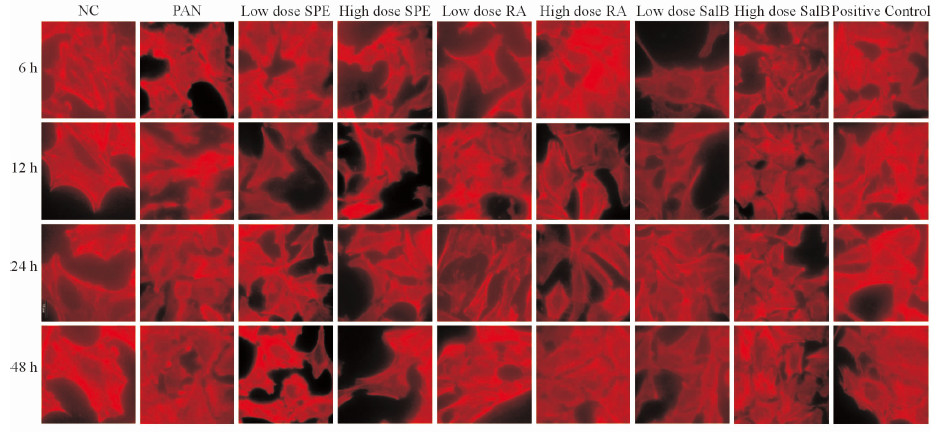
|
图 4 各组各时间点小鼠足细胞F-actin骨架蛋白染色 Fig 4 Staining of F-actin of mouse podocytes of each group at different time points vessels NC: Normal control; PAN: Puromycin aminonucleoside (100 μg/mL); Low dose SPE: SPE (158 μg/mL); High dose SPE: SPE (316 μg/mL); Low dose RA: RA (25 μg/mL); High dose RA: RA (50 μg/mL); Low dose SalB: SalB (8.5 μg/mL); High dose SalB: SalB (17 μg/mL); Positive control: Tacrolimus (1 μg/mL). SPE: Salvia przewalskii extract of total phenolic acids; RA: Rosmarinic acid; SalB: Salvianolic acid B. Original magnification: ×1 000 |
相比于正常组,PAN作用足细胞24 h后足细胞内ROS荧光强度增加(P<0.05)。各时间点模型对照组和阳性对照组间差异有统计学意义(P<0.05),其中24 h SPE、SalB及RA高、低剂量组组间差异有统计学意义(P<0.05),说明SPE及其单体可以有效降低足细胞内ROS荧光强度,且降低程度与药物剂量相关。而阳性对照组与SPE低剂量组、SalB和RA高剂量组间差异无统计学意义(P>0.05),提示SPE低剂量组、SalB和RA高剂量组与阳性对照组降低足细胞内ROS荧光强度效果较接近。SalB高、 低剂量组分别与RA高、低剂量组足细胞间ROS荧光强度比较差异有统计学意义(P<0.05),说明SalB组降低足细胞内ROS荧光强度效果优于RA组(图 5)。
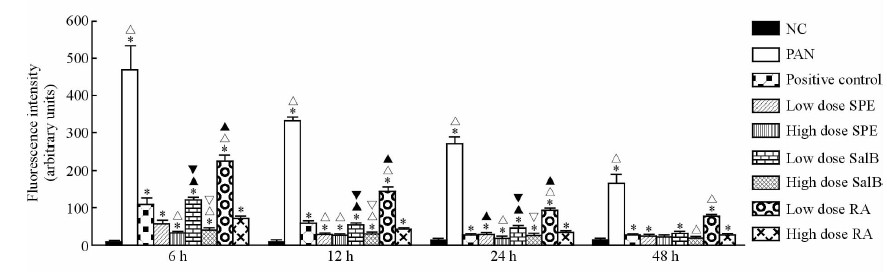
|
图 5 各组小鼠足细胞各时间点ROS荧光强度 Fig 5 Fluorescence intensities of ROS of mouse podocytes of each group at different time points vessels NC: Normal control; PAN: Puromycin aminonucleoside (100 μg/mL); Low dose SPE: SPE (158 μg/mL); High dose SPE: SPE (316 μg/mL); Low dose RA: RA (25 μg/mL); High dose RA: RA (50 μg/mL); Low dose SalB: SalB (8.5 μg/mL); High dose SalB: SalB (17 μg/mL); Positive control: Tacrolimus (1 μg/mL). SPE: Salvia przewalskii extract of total phenolic acids; RA: Rosmarinic acid; SalB: Salvianolic acid B. *P<0.05 vs NC; △P<0.05 vs positive control; ▲P<0.05 vs the same group of high dose; ▽P<0.05 vs high dose RA; ▼P<0.05 vs low dose RA. n=3, x±s |
足细胞是高度分化的细胞,可以防止尿蛋白渗漏,维持肾小球内毛细血管襻完整性[19],在肾小球滤过屏障中起着至关重要的作用[20]。既往研究证实,PAN诱导足细胞超微结构发生改变,肾小球基底膜脱落、细胞骨架解聚[21]、肾小球基底膜大小及电荷损伤[22]。
我们前期体内研究同样发现PAN诱导足细胞损伤后,电镜下观察到足细胞足突在第10天时融合消失。此外,我们通过免疫组化、蛋白质免疫印迹和RT-PCR分别从蛋白和mRNA水平研究发现PAN肾病大鼠模型足细胞裂孔膜蛋白nephrin和podocin的表达下调[6]。
肾小球足细胞数常常通过计数足细胞核来完成,WT1是一种锌指样转录因子,定位于成熟足细胞核,是足细胞特异性标志之一[23, 24, 25, 26, 27, 28]。本研究发现,PAN注射后大鼠WT1阳性足细胞数目减少,低于正常组。给予SPE和他克莫司治疗后,治疗组各时间点足细胞数量高于模型对照组,其中,SPE高剂量组与阳性对照组保护足细胞效果较为接近。此外,体外研究发现PAN几乎能完全破坏足细胞的骨架结构,使F-actin的骨架丝状结构消失,进一步证实PAN对足细胞具有损伤作用。在给予SPE、SalB、RA和他克莫司后,PAN诱导的足细胞损伤减弱,细胞内重新出现极性分布的微丝,在体内和体外进一步证明SPE具有保护足细胞作用。
PAN造成足细胞损伤的机制可能是由于足细胞受到损伤,产生过多的ROS所致[29]。ROS可参与多种信号转导通路,介导细胞分化、凋亡及增殖[30]。Kawaguchi等[31]研究发现PAN刺激足细胞后,细胞扁平变圆,部分脱离培养皿,投射电镜观察到细胞胞质空泡及破裂的气球样空间,添加抗氧化酶后细胞扁平及变圆程度减轻,表明抗氧化剂对足细胞具有保护作用。
文献报道,甘西鼠尾草能够降低脂质过氧化物丙二醛的含量,抑制羟自由基的产生,抑制脂质过氧化反应;SPE及其单体具有抑制超氧自由基及抗氧化、抑制心肌缺血再灌注损伤等作用[32],因而,我们推测SPE对足细胞的保护作用也可能通过上述的抗氧化应激通路实现。他克莫司是公认的保护足细胞药物,Yousuf等[33]在大鼠缺氧损伤模型中证实他克莫司具有抗氧化应激损伤作用。因而,本研究采用他克莫司作为阳性对照观察SPE及其单体对足细胞氧化损伤的保护作用。
8-OHdG是活性氧类物质如羟自由基、单线态氧等攻击DNA分子中的鸟嘌呤碱基第8位碳原子所致的核苷酸损伤物[34]。在各种碱基修饰产物中,8-OHdG被公认为DNA氧化损伤的重要标志物[16],其只通过DNA氧化损伤途径形成,非细胞更新产物,不会在系统循环中由鸟嘌呤氧化而成,为代谢终物,在体内稳定存在[35];其在组织细胞核DNA中的含量可以反映体内特定部位DNA氧化损伤和机体的正常修复作用。通过测定大鼠肾脏组织中8-OHdG的荧光强度,本研究发现模型对照组大鼠足细胞中8-OHdG荧光强度增强,证实PAN诱导足细胞损伤后对DNA造成损伤,产生过多的8-OHdG。SPE干预后可以有效降低大鼠肾组织中8-OHdG的荧光强度,且SPE高剂量组相比中、低剂量组效果较为明显,与阳性对照组效果相似。 此外,体外研究发现PAN作用足细胞24 h后,足细胞内ROS荧光强度较正常组增加,给予SPE干预后,发现各时间点模型对照组和药物治疗组间差异有统计学意义;此外,SalB高、低剂量分别组与RA高、低剂量组比较差异也有统计学意义,提示SalB组效果优于RA组。结果表明SPE及其单体可以有效抑制PAN所致足细胞氧化应激损伤,从而对足细胞具有保护作用。
本研究从体内及体外证实,SPE对PAN所致的足细胞氧化损伤具有保护作用。然而,SPE对足细胞保护作用的具体机制仍需进一步研究和探讨。本课题组拟下一步研究SPE及其单体是否对PAN诱导的小鼠足细胞内线粒体、凋亡机制以及Toll样受体通路产生保护作用,以期进一步明确SPE保护足细胞的作用机制,从而为临床治疗足细胞病提供一条新的治疗途径。
| [1] | 姜爱莉, 孙丽芹, 王 文, 翁新楚.四种植物天然抗氧化剂活性初探[J]. 食品科学, 2002, 23:35-38. |
| [2] | Kasimu R, Tanaka K, Tezuka Y, Gong Z N, Li J X, Basnet P, et al. Comparative study of seventeen Salvia plants: aldose reductase inhibitory activity of water and MeOH extracts and liquid chromatography-mass spectrometry(LC-MS) analysis of water extracts[J]. Chem Pharm Bull (Tokyo), 1998, 46: 500-504. |
| [3] | 李 霞, 杨 阳, 黄豆豆, 黄光辉, 孙连娜.甘西鼠尾草药材和提取物中迷迭香酸、丹酚酸 B 的含量测定[J].中国医院药学杂志, 2014, 34:1634. |
| [4] | 孙连娜, 陈万生, 杨 阳, 张 凤, 来 威, 曹永兵, 等.甘西鼠尾草提取物在制备治疗肾小球肾炎药物中的应用: 中国, 200710048139.3[P]. 2008-05-21. |
| [5] | 杨 阳, 朱 斌, 吴志军, 陈万生, 孙连娜.甘西鼠尾草提取物对正常大鼠全血粘度影响及对水负荷大鼠利尿作用[J].中国医院药学杂志, 2012, 32:751-754. |
| [6] | Dai D S, Liu X, Yang Y, Luo X M, Tang R X, Yin Z C, et al. Protective effect of Salvia przewalskii extract on puromycin-induced podocyte injury[J]. Am J Nephrol, 2015, 42: 216-227. |
| [7] | Gwinner W, Landmesser U, Brandes R P, Kubat B, Plasger J, Eberhard O, et al. Reactive oxygen species and antioxidant defense in puromycin aminonucleoside glomerulopathy[J]. J Am Soc Nephrol, 1997, 8: 1722-1731. |
| [8] | Beaman M, Birtwistle R, Howie A J, Michael J, Adu D. The role of superoxide anion and hydrogen peroxide in glomerular injury induced by puromycin aminonucleoside in rats[J]. Clin Sci (Lond), 1987, 73: 329-332. |
| [9] | Shibouta Y, Terashita Z, Imura Y, Shino A, Kawamura M, Ohtsuki K, et al. Involvement of thromboxane A2, leukotrienes and free radicals in puromycin nephrosis in rats[J]. Kidney Int, 1991, 39: 920-929. |
| [10] | Ricardo S D, Bertram J F, Ryan G B.Antioxidants protect podocyte foot processes in puromycin aminonucleoside-treated rats[J]. J Am Soc Nephrol, 1994, 4: 1974-1986. |
| [11] | Nakamura K, Kojima K, Arai T, Shirai M, Usutani S, Akimoto H, et al. Dipyridamole and dilazep suppress oxygen radicals in puromycin aminonucleoside nephrosis rats[J]. Eur J Clin Invest, 1998, 28: 877-883. |
| [12] | Cooke M S, Evans M D, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease[J]. FASEB J, 2003, 7: 1195-1214. |
| [13] | Ece H, Cigdem E, Yuksel K, Ahmet D, Hakan E, Oktay T M. Use of oral antidiabetic drugs (metformin and pioglitazone) in diabetic patients with breast cancer: how does it effect serum Hif-1α and 8OHdG levels [J].Asian Pac J Cancer Prev, 2012, 13: 5143-5148. |
| [14] | Yang Y, Bing Z, Sun L, Wu Z, Chen W. Chemical constituents of Salvia przewalskii Maxim.[J].Asian J Chem, 2013, 25: 1747-1748. |
| [15] | Kim Y H, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, et al.Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat[J]. Kidney Int, 2001, 60: 957-968. |
| [16] | Guo K, Lu J, Huang Y, Wu M, Zhang L, Yu H, et al. Protective role of PGC-1α in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling[J]. PLoS One, 2015, 10: e0125176. |
| [17] | Mundel P, Reiser J, Zúňiga Mejía Borja A, Pavenstädt H, Davidson G R, Kriz W, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines[J]. Exp Cell Res, 1997, 236: 248-258. |
| [18] | Liu Z, Zhao W, Zhang Q, Lai L, Jiang S, Zhang J, et al. Increased oxidative damage and reduced DNA repair enzyme XPD involvement in high glucose-mediated enhancement of levobupivacaine-induced neurotoxicity[J]. Neurochem Res, 2015, 40: 1919-1928. |
| [19] | Kriz W, Hackenthal E, Nobiling R, Sakai T, Elger M, Hähnel B. A role for podocytes to counteract capillary wall distension[J]. Kidney Int, 1994, 45: 369-376. |
| [20] | Pavenstädt H. Roles of the podocyte in glomerular function[J]. Am J Physiol Renal Physiol, 2000, 278: F173-F179. |
| [21] | Whiteside C I, Cameron R, Munk S, Levy J. Podocytic cytoskeletal disaggregation and basement-membrane detachment in puromycin aminonucleoside nephrosis[J]. Am J Pathol, 1993, 142: 1641-1653. |
| [22] | Garin E H, Shirey A J. Glomerular basement membrane heparan sulfate glycosaminoglycan in aminonucleoside of puromycin nephrosis[J]. Child Nephrol Urol, 1988, 9: 121-126. |
| [23] | Morrison A A, Viney R L, Saleem M A, Ladomery M R. New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes[J]. Am J Physiol Renal Physiol, 2008, 295: F12-F17. |
| [24] | Wagner K D, Wagner N, Schedl A. The complex life of WT1[J]. J Cell Sci, 2003, 116(Pt 9): 1653-1658. |
| [25] | Palmer R E, Kotsianti A, Cadman B, Boyd T, Gerald W, Haber D A. WT1 regulates the expression of the major glomerular podocyte membrane protein podocalyxin[J]. Curr Biol, 2001, 11: 1805-1809. |
| [26] | Guo J K, Menke A L, Gubler M C, Clarke A R, Harrison D, Hammes A, et al. WT1 is a key regulator of podocyte function: reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis[J]. Hum Mol Genet, 2002, 11: 651-659. |
| [27] | Guo G, Morrison D J, Licht J D, Quaggin S E. WT1 activates a glomerular-specific enhancer identified from the human nephrin gene[J]. J Am Soc Nephrol, 2004, 15: 2851-2856. |
| [28] | Wagner N, Wagner K D, Xing Y, Scholz H, Schedl A. The major podocyte protein nephrin is transcriptionally activated by the Wilms' tumor suppressor WT1[J]. J Am Soc Nephrol, 2004, 15: 3044-3051. |
| [29] | Marshall C B, Pippin J W, Krofft R D, Shankland S J. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo[J]. Kidney Int, 2006, 70: 1962-1973. |
| [30] | Rojas F, Gonzalez D, Cortes N, Ampuero E, Hernández D E, Fritz E, et al. Reactive oxygen species trigger motoneuron death in non-cell-autonomous models of ALS through activation of c-Abl signaling[J]. Front Cell Neurosci, 2015, 9: 203. |
| [31] | Kawaguchi M, Yamada M, Wada H, Okigaki T. Roles of active oxygen species in glomerular epithelial cell injury in vitro caused by puromycin aminonucleoside[J]. Toxicology, 1992, 72: 329-340. |
| [32] | 杨 阳, 张 凤, 蔡 飞, 孙连娜, 陈万生.甘西鼠尾草化学成分及药理作用研究进展[J].中药材, 2008, 31: 787-790. |
| [33] | Yousuf S, Atif F, Kesherwani V, Agrawal S K. Neuroprotective effects of tacrolimus (FK-506) and cyclosporin (CsA) in oxidative injury[J]. Brain Behav, 2011, 1: 87-94. |
| [34] | Cooke M S, Evans M D, Dove R, Rozalski R, Gackowski D, Siomek A, et al. DNA repair is responsible for the presence of oxidatively damaged DNA lesions in urine[J]. Mutat Res, 2005, 574: 58-66. |
| [35] | Wu L L, Chiou C C, Chang P Y, Wu J T. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics[J]. Clin Chim Acta, 2004, 339: 1-9. |
 2016, Vol. 37
2016, Vol. 37


