2. 潍坊诸城中医医院肿瘤科, 潍坊 262200
2. Department of Oncology, Weifang Zhucheng Traditional Chinese Medicine Hospital, Weifang 262200, Shandong, China
胰腺癌是常见的消化系统肿瘤之一,占世界恶性肿瘤发病率的第10位(3%),而死亡率为第4位(7%),发病率及死亡率在人群中均呈上升趋势[1]。胰腺癌起病隐匿,早期诊断率低,恶性程度高,手术切除率低,对放疗、化疗不敏感,临床预后极差,5年生存率低于6%,为唯一发病率与病死率相当的恶性肿瘤[1]。局部进展期及转移性胰腺癌约占全部胰腺癌的80%,局部晚期中位生存期为6~10个月,转移性胰腺癌中位生存期仅3~6个月[1]。对于局部晚期和转移性胰腺癌,局部消融治疗可达到消灭肿瘤、减轻疼痛、延长患者生存期的目的。目前国内常见的消融治疗主要有射频消融 (radiofrequency ablation,RFA)、微波消融(microwave ablation,MWA)、冷冻消融 (cryoablation) 和高强度聚焦超声(high-intensity focused ultrasound,HIFU),且被证实可延长局部晚期及转移性胰腺癌患者生存期,但是受到适应证、禁忌证和不良反应等因素的限制,治疗效果仍不理想。 不可逆电穿孔(irreversible electroporation,IRE,俗称“纳米刀”)治疗肿瘤是通过释放 kV 级高压的直流电,以电脉冲方式产生强力的电场,在细胞膜上产生永久性的纳米孔,促进肿瘤细胞凋亡,消融肿瘤组织。本研究选取新西兰大白兔VX2 胰腺移植瘤为研究对象,探究纳米刀、冷冻和射频消融除直接消融肿瘤组织外的其他抗肿瘤机制,同时探讨纳米刀在治疗胰腺移植瘤的安全性和有效性。
1 材料和方法 1.1 新西兰大白兔VX2 胰腺移植肿瘤模型建立新西兰大白兔8只,体质量为2.1~3.4 kg,购自上海斯莱克实验动物中心 [动物生产许可证号: SCXK(沪)2014-0008]。氯胺酮4~6 mg/kg肌肉注射麻醉新西兰大白兔,左上腹部中线旁行1cm的纵行切口,将1 mm3瘤块植入胰体尾处,术后3 d内常规肌肉注射抗生素,生长2周后可见直径约2 cm肿瘤结节(图 1A)。

|
图 1 各组实验兔胰腺移植瘤大体(A-D)及组织病理学(E-I)结果 Fig 1 Gross specimen (A-D) and histopathological results (E-I) of pancreatic cancer xenografts in rabbits of each group A: Gross specimen of the rabbit pancreatic xenograft, with rich angiogenesis visible on the surface of tumor and adhered to the surrounding tissue; B-D: The schematic illustrations of irreversible electroporation (IRE), cryoablation, and radiofrequency ablation(RFA)treatment of rabbit pancreatic xenografts; E: In the control group, tumor cells were arranged closely, with markedly varied morphology, hemorrhage and necrosis; F: After IRE, tumor cell nuclei were compressed, and fragmentized or dissolved; G: Ablation area boundary was irregular, and some survival cells could be seen in the ablation area; H: Clear border was seen between the treatment area and the untreatment area in the RFA group, with the necrosis area filled with red dye fragments; I: A large number of apoptotic cells could be seen in the RFA area. Original magnification:×400(E-I) |
将实验兔分为模型对照组、纳米刀组、冷冻组、射频组,每组2只。纳米刀治疗过程中全麻配合气管插管(氯胺酮0.1~0.15 mg/只,配合肌松药氯化琥珀胆碱0.1~0.2 mg/只),术后简易呼吸器复苏,余治疗组以氯胺酮0.1~0.15mg/只麻醉,麻醉满意后开腹暴露胰腺移植瘤,将电极插入肿瘤组织内。纳米刀实验参数:AngioDynamics公司制造的纳米刀正负两电极,正负电极之间的距离为1.0~1.5 cm,进针深度为1.0~1.5 cm,电压1 800~2 400 V/cm,脉冲长度70/s,脉冲数目10,脉冲时值90 μs (图 1B)。冷冻参数:采用美国氩氦刀冷冻系统进行治疗,冷冻探针为 1.47 mm,冷冻消融肿瘤模式为:氩气急剧降温冷冻 5 min,氦气快速复温 2 min为1个循环,共 2个冷冻/复温循环 (图 1C) 。 射频参数:采用RF-2000射频消融仪及MEDSPHERE电外科电极,将 MEDSPHERE电外科电极针准确插入病灶内,射频输出功率150 W,治疗总时间为8~13 min (图 1D)。
1.3 样本处理及检测所有实验兔术后24 h处死,取血,分析血清和血浆,各组标本经甲醛固定处理。血清进行肝肾功能、淀粉酶(AMY)、肌酸激酶检查,血浆标本采用酶标仪双抗体夹心 (ELISA ) 法测定心肌肌钙蛋白I(CTnI)、Caspase-3、肿瘤坏死因子(TNF)-α及血管内皮生长因子(vascular endothelial growth factor,VEGF) 的生物学活性。H-E染色后光镜下观察各组术后移植瘤组织的形态学改变,同时Tunnel试剂染色,共聚焦显微镜下拍照。采用Envision法进行 Bcl-2、VEGF、HSP70 免疫组化染色,所用的一抗为:兔抗兔多克隆抗体 Bcl-2(工作浓度为 1∶1 000,台湾 Abnova公司,货号:PAB19562);鼠抗兔 HSP70单克隆抗体(工作浓度为 1∶1 000,美国 Abcam 公司,货号:ab2787)、鼠抗兔 VEGF多克隆抗体(工作浓度为 1∶1 000,美国 Abcam 公司,货号:ab1316)。HSP70、Bcl-2及VEGF染色阳性信号均呈棕黄色颗粒,HSP70、VEGF 表达定位于细胞质,Bcl-2 表达定位于细胞核及细胞质。
1.4 统计学处理采用Graphpad prim 5和Excel软件进行图形绘制,Adobe Photoshop软件进行图形编辑。采用SPSS 19.0软件进行数据分析,两组数据间比较采用t检验,检验水准(α)为0.05。
2 结 果 2.1 术后大体标本及组织病理学观察对照组实验兔脾胃间隙间可见直径约2 cm结节,肿瘤表面呈灰红色,质偏软,表面可见丰富的供瘤血管(图 1A);镜下见癌细胞密集,间质少,核大染色深,其大小和形态各异,染色浓淡不均,异常分裂像多见,细胞呈不规则排列,新生毛细血管丰富,肿瘤中心可见出血坏死(图 1E)。
纳米刀组消融区边界清晰,核固缩、碎裂,蓝染;肿瘤血管变性坏死、血栓形成,内见红细胞碎裂物沉积,但血管腔结构完整(图 1F)。
冷冻组肿瘤组织充血水肿,无光泽,触之较硬;镜下见细胞完全坏死崩解、核碎裂,呈凝固性坏死,坏死区域周边(冰球边缘)出现明显的损伤区带,其中仍可见部分癌细胞,但细胞皱缩,核浓集,染色质固缩,呈典型的凋亡细胞特征(图 1G)。
射频组消融区中央完全坏死,见大片无定结构的粉染区,其周边为凝固性坏死;镜下见细胞核固缩、碎裂和溶解,内仍可见部分存活肿瘤细胞(图 1H),术区边缘血管充血,可见大量凋亡细胞(图 1I)。
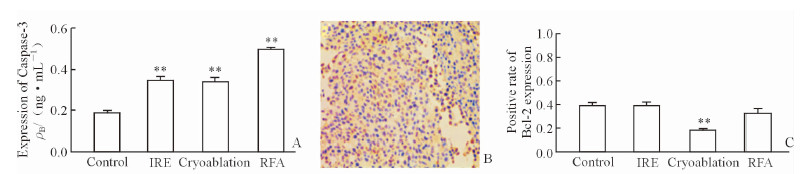
2.2 肿瘤凋亡相关因子变化Bcl-2家族是重要的凋亡相关因子,激活线粒体及内质网的内外凋亡通路发挥促肿瘤组织凋亡,Bax(促凋亡蛋白)/ Bcl-2(促生存蛋白)的比值升高,促进Caspase-3表达,细胞接受凋亡信号刺激发生不可逆性凋亡。本实验血浆ELISA结果显示,各治疗组术后机体Caspase-3水平均较对照组不同程度升高(P < 0.01,图 2A)。Bcl-2主要表达于胞质,部分可见胞核浓染(图 2B);与对照组比较,纳米刀治疗组及射频治疗组Bcl-2表达差异无统计学意义,而冷冻治疗组Bcl-2表达低于对照组(P < 0.01,图 2C)。
|
图 2 各组间肿瘤凋亡相关因子Caspase-3、Bcl-2的表达 Fig 2 Tumor apoptosis related factor Caspase-3 and Bcl-2 expression in the each group A: Plasma Caspase-3 levels in each group; B: Bcl-2 expression in border of irreversible electroporation (IRE) treatment area; yellow dye could be seen in the cell cytoplasm and cell nucleus (Envision method, original magnification:×200); C: Positive rate of Bcl-2 expression in each group.RFA: Radiofrequency ablation. **P < 0.01 vs control group. n=6, x±s |
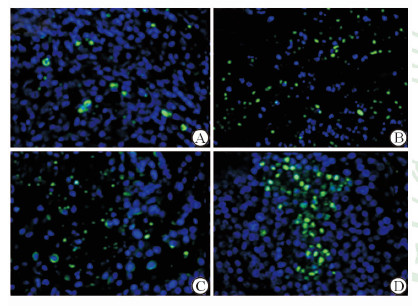
Tunnel方法又称DNA断裂的原位末端标记法,是用来检测组织细胞在凋亡早期过程中细胞核DNA的断裂情况,特异准确地定位正在凋亡的细胞。本实验各组消融区边缘肿瘤细胞可见典型Tunnel阳性细胞,有的散在分布见,有的集中分布(图 3),其中射频治疗组凋亡率最高 (图 3D) 。
|
图 3 各组消融区边缘细胞凋亡情况 Fig 3 Apoptosis in ablation marginal cells after treatment in each group A: Control group; B:Irreversible electroporation group; C: Cryoablation group; D: Radiofrequency ablation group. Original magnification: × 200 |
TNF-α是多效的促炎细胞因子,介导机体免疫调节功能。HSP70是热休克蛋白家族中最保守、最重要的一族,在肿瘤免疫中发挥重要作用。各治疗组血浆TNF-α水平及术区边缘(除冰冻治疗组)HSP70表达量均较模型对照组升高(P < 0.01),尤以纳米刀治疗组最明显(图 4)。

|
图 4 各组血浆TNF-α和术区边缘HSP70的表达水平 Fig 4 Expression of plasma TNF-α and HSP70 in the border of treatment area in each group A: Variation of plasma TNF-α level in each group; B: HSP70 expression in the border of irreversible electroporation (IRE) treatment area, with yellow dye seen in the cell cytoplasm and nucleus (Envision method, original magnification: ×200); C: The positive rate of HSP70 expression in each group. RFA: Radiofrequency ablation.**P < 0.01 vs control group. n=6, x±s |
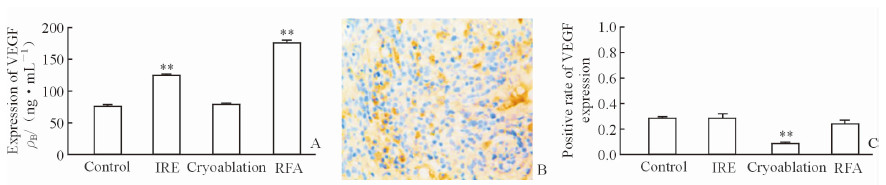
VEGF 是一种功能最强的促血管生成因子,与肿瘤的侵袭、转移等生物学特性密切相关。各治疗组血浆VEGF水平均有所升高,以射频治疗组为著。术区VEGF 淡染,以肿瘤边缘为主,冷冻治疗组VEGF下降最为明显,纳米刀治疗组及射频治疗组未见明显降低(图 5)。
|
图 5 各组实验兔术后血浆及术区VEGF的表达水平 Fig 5 Post-treatment plasma and treatment area VEGF expression levels in rabbits of each group A: Variation of plasma VEGF level in rabbits; B: VEGF expression in the edge of IRE treatment area, with yellow dye seen in the cell nucleus and partial cell cytoplasm (Envision method, original magnification: × 200); C: The positive rate of VEGF expression in each group.IRE:Irreversible electroporation; RFA: Radiofrequency ablation; VEGF: Vascular endothelial growth factor. **P < 0.01 vs control. n=6, x±s |
纳米刀治疗过程中实验兔伴随脉冲作用出现脉冲式肌肉强直收缩,射频及冷冻消融组治疗时未见异常。由表 1可见,纳米刀治疗组实验兔出现肌酸激酶升高,冷冻治疗组及射频治疗组出现谷草转氨酶(AST)轻度升高;不同术式术后血清AMY均出现不同程度升高;肝肾功能未见明显异常。
|
|
表 1 各组肝肾功能、淀粉酶、肌酸激酶及肌钙蛋白I变化 Tab 1 Variations of postoperative liver and kidney function, AMY, CK-MB and CTnI levels in each group |
目前晚期胰腺癌主要治疗手段仍是化疗,各种化疗方案对胰腺癌患者的总体生存期未见明显改善[2, 3, 4],包括吉西他滨联合卡培他滨(中位生存期为7.1个月)、FOLFIRINOX(中位生存期为11.1个月)及白蛋白紫杉醇联合吉西他滨(总体生存期为8.5个月),所以胰腺癌治疗仍面临巨大挑战。积极寻找其他替代治疗方式及方案对改善胰腺癌患者生存期和生活质量具有重要的意义。既往晚期胰腺肿瘤局部冷冻、射频作为一种姑息性治疗方式取得一定的理想效果,但是由于临近腹腔大血管及胆道系统,存在出血、胆漏及胰漏风险,而且其具体参数的设置及操作要点尚需更进一步的研究和探讨[5, 6, 7]。纳米刀作为非热效应的一种新兴微创治疗手段,大大减少了胰腺癌治疗导致的消化道出血及胆道损伤,发展前景广阔[8, 9, 10]。本研究通过抗肿瘤的安全性和有效性探讨纳米刀在治疗不可切除性胰腺癌的优势。
本实验结果发现,纳米刀治疗组的血管结构未见明显破坏,内可见多量破碎红细胞沉积;纳米刀灭活区域和非灭活区域之间存在明显分界线,而冷冻或射频等其他热灭活方法则产生一片无定形区域,这片区域很有可能存在不完全灭活的组织而造成部分肿瘤细胞残余或肿瘤复发[11]。这一结果与纳米刀破坏细胞膜的完整性、继而发生细胞凋亡或坏死、但不影响由蛋白质为主要构成成分的组织框架结构[12]有关,钠米刀有可能成为治疗毗邻神经、血管等重要敏感组织肿瘤(晚期胰腺癌、邻近门静脉及胆道的肝恶性肿瘤结节)的一种有效方法。
纳米刀、冷冻及射频消融除能直接毁损肿瘤组织外,还均能通过促肿瘤组织凋亡、增强机体免疫及抑制肿瘤侵袭转移等机制发挥抗肿瘤作用,但存在细微的差别,这与三者的消融机制不同有关:纳米刀通过一定的电脉冲剂量使细胞膜出现不可恢复的破裂,导致细胞死亡[13];冷冻消融是先通过冷冻使癌细胞内形成冰晶,再快速升温使细胞内的冰晶爆裂,癌细胞被完全摧毁,通过这种快速制冷和急速升温来抗肿瘤[14]。射频消融通过一定的射频输出,使靶区组织细胞离子振荡摩擦产生热量,局部温度可达80℃~90℃,足以使肿瘤组织产生凝固性坏死,并最终形成液化灶或纤维组织,达到治疗肿瘤目的[15]。
Bcl-2 是 Bcl-2 家族重要的促生存蛋白因子,Bcl-2 的降低促发肿瘤凋亡[16],同时促进 Caspase-3这一重要促凋亡因子的表达,进而导致肿瘤细胞发生不可逆性凋亡[17]。本实验血浆 ELISA 结果显示,与对照组比较,各治疗组Caspase-3水平不同程度升高,射频治疗组消融范围较其余治疗组显著,而冷冻治疗组的Bcl-2下降最为明显,与消融区Tunnel结果一致,这与各组治疗机制不同有关[13, 14, 15]。刺激机体的免疫发挥抑瘤的后遗效应,是微创治疗的另一重要抗肿瘤机制。TNF-α和 HSP70 在肿瘤免疫中发挥重要作用,能诱导和增强机体抗肿瘤免疫反应,抑制肿瘤生长[18, 19]。本实验结果显示,纳米刀治疗后可激发机体自身免疫系统,发挥抗肿瘤的后遗效应,这与纳米刀消融迅速、完全彻底、大量释放肿瘤抗原碎片、激发机体免疫系统有关。肿瘤的重要特征之一是不同阶段发生侵袭转移,VEGF 是其中重要的启动子之一,肿瘤血管生成的程度间接反映了肿瘤的浸润、复发及转移的能力[20]。本研究各治疗组血浆VEGF因子水平较对照组升高,可能与肿瘤血管破坏后短时间内VEGF 释放入血有关,术区VEGF 表达有所下降尤以冷冻治疗组为著,这与冷冻消融的“冷冻栓塞”有关[21],表明各治疗组能一定程度抑制血管形成因子的表达,减少肿瘤组织的血液供应,抑制癌细胞的迁移,可能是消融技术治疗肿瘤的一项重要机制。
在安全性方面,血清检测结果显示,与对照组相比,纳米刀组肌酸激酶水平升高,这可能与实验兔治疗过程中的肌肉抽搐有关。冷冻及射频治疗后邻近粘连的小肠明显充血水肿,而纳米刀组未见明显肠道损伤,充分说明纳米刀消融区的边界清晰。因此,与冷冻及射频治疗相比,纳米刀更适合进展期胰腺癌的局部减瘤治疗,尤其是包绕血管、胆管及神经等重要结构时,能有效地保存重要结构,预防严重并发症的发生[22]。
本组实验中,纳米刀治疗组在激发机体免疫和保护周围重要脏器方面有一定的优势。但是由于本研究实验样本数较少,纳米刀对比于冷冻及射频消融在胰腺癌中的应用优势、治疗各项参数的优化及选择还需更多临床前研究。
| [1] | Siegel R L, Miller K D, Jemal A.Cancer statistics, 2015[J].CA Cancer J Clin,2015,65:5-29. |
| [2] | Cunningham D, Chau I, Stocken D D, Valle J W, Smith D, Steward W,et al.Phase Ⅲ randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer[J].J Clin Oncol,2009,27:5513-5518. |
| [3] | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup.FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer[J].N Engl J Med,2011,364:1817-1825. |
| [4] | Yardley D A, Brufsky A, Coleman R E, Conte P F, Cortes J, Glück S,et al.Phase Ⅱ/Ⅲ weekly nab-paclitaxel plus gemcitabine or carboplatin versus gemcitabine/carboplatin as first-line treatment of patients with metastatic triple-negative breast cancer (the tnAcity study): study protocol for a randomized controlled trial[J].Trials,2015,16:575. |
| [5] | Niu L, He L, Zhou L, Mu F, Wu B, Li H,et al.Percutaneous ultrasonography and computed tomography guided pancreatic cryoablation: feasibility and safety assessment[J].Cryobiology,2012,65:301-307. |
| [6] | Fegrachi S, Besselink M G, van Santvoort H C, van Hillegersberg R, Molenaar I Q.Radiofrequency ablation for unresectable locally advanced pancreatic cancer: a systematic review[J].HPB (Oxford),2014,16:119-123. |
| [7] | Girelli R, Frigerio I, Giardino A, Regi P, Gobbo S, Malleo G, et al.Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage Ⅲ ductal adenocarcinoma[J].Langenbecks Arch Surg,2013,398:63-69. |
| [8] | Rubinsky B, Onik G, Mikus P.Irreversible electroporation: a new ablation modality-clinical implications[J].Technol Cancer Res Treat,2007,6:37-48. |
| [9] | Au J T, Kingham T P, Jun K, Haddad D, Gholami S, Mojica K, et al.Irreversible electroporation ablation of the liver can be detected with ultrasound B-mode and elastography[J].Surgery,2013,153:787-793. |
| [10] | Kingham T P, Karkar A M, D'Angelica M I, Allen P J, Dematteo R P, Getrajdman G I, et al.Ablation of perivascular hepatic malignant tumors with irreversible electroporation[J].J Am Coll Surg,2012,215:379-387. |
| [11] | Yu Z, Zhang X, Ren P, Zhang M, Qian J.Therapeutic potential of irreversible electroporation in sarcoma[J].Expert Rev Anticancer Ther,2012,12:177-184. |
| [12] | Lee E W, Chen C, Prieto V E, Dry S M, Loh C T, Kee S T.Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation[J].Radiology,2010,255:426-433. |
| [13] | Weaver J C. Electroporation: a dramatic, non-thermal electric field phenomenon[C] //Proceeding of the First World Congress for Electricity and Magnetism in Biology and Medicine.Lake Buena Vista. Florida: Academic Press, 1992:14. |
| [14] | 徐克成, 牛立志. 肿瘤冷冻治疗学[M]. 上海: 上海科技教育出版社, 2007: 218-225. |
| [15] | Fegrachi S, Molenaar I Q, Klaessens J H, Besselink M G, Offerhaus J A, van Hillegersberg R.Radiofrequency ablation of the pancreas with and without intraluminal duodenal cooling in a porcine model[J].J Surg Res,2013,184:867-872. |
| [16] | 邵佳亮,胡国信,郑 洁,万赞燕,姜瑞娇.羟基喜树碱对肝纤维化大鼠肝组织Bax、Bcl-2基因和α-SMA蛋白表达及肝纤维化的影响[J]. 第二军医大学学报, 2014, 35: 399-405. SHAO J L, HU G X, ZHENG J, WAN Z Y, JIANG R J.Effects of hydroxycamptothecin on hepatic expression of Bax and Bcl-2 genes and α-SMA protein and hepatic fibrosis in rats[J]. Acad J Sec Mil Med Univ, 2014,35:399-405. |
| [17] | 赵 瑞,唐春花,赵文元,刘建民,洪 波,许 奕,等.Caspase-3体外对PIAS1蛋白酶切作用的初步验证[J]. 第二军医大学学报,2011, 32: 1144-1146. ZHAO R, TANG C H, ZHAO W Y, LIU J M, HONG B, XU Y, et al. Cleavage effect of Caspase-3 on PIAS1 protein in vitro[J]. Acad J Sec Mil Med Univ,2011,32:1144-1146. |
| [18] | 付清松, 夏玉军, 张 明, 刘 明, 单守勤.三维球体间充质干细胞移植对大鼠缺血再灌注损伤脑组织TNF-α及凋亡相关蛋白表达的影响[J]. 第二军医大学学报, 2015, 36: 845-850. FU Q S, XIA Y J,ZHANG M, LIU M, SHAN S Q.Transplantation with three-dimensional spheroid-cultured mesenchymal stem cells down-regulates expression of TNF-α and apoptosis-related proteins in rats with cerebral ischemia/reperfusion injury[J]. Acad J Sec Mil Med Univ,2015,36:845-850. |
| [19] | Tsan M F, Gao B.Heat shock proteins and immune system[J].J Leukoc Biol,2009,85:905-910. |
| [20] | Das M, Wakelee H.Targeting VEGF in lung cancer[J].Expert Opin Ther Targets,2012,16:395-406. |
| [21] | Zhen W, Gang Z, Tao W, Qian F Y, Min Y S, Xiao M H. Three-dimensional numerical simulation of the effects of fractal vascular trees on tissue temperature and intracelluar ice formation during combined cancer therapy of cryosurgery and hyperthermia[J]. Applied Thermal Engineering, 2015, 90: 296-304. |
| [22] | Hildebrand P, Leibecke T, Kleemann M, Mirow L, Birth M, Bruch H P,et al.Influence of operator experience in radiofrequency ablation of malignant liver tumours on treatment outcome[J].Eur J Surg Oncol,2006,32:430-434. |
 2016, Vol. 37
2016, Vol. 37


