2. 第二军医大学长海医院药学部, 上海 200433;
3. 上海交通大学医学院附属仁济医院中心实验室, 上海 200127
2. Department of Pharmacy, Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
3. Central Laboratory, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200127, China
肿瘤的发生发展多与炎症密切相关,如乙肝病毒感染导致的肝脏炎症与肝癌[1, 2]、慢性胆囊炎和胆结石与胆囊癌的发生[3, 4]、幽门螺旋杆菌(HP)所导致的胃溃疡与胃癌等[5, 6]。结肠癌(colon cancer)作为人类高发的恶性肿瘤之一,其发生发展过程中也多伴随炎症反应,炎症性肠病(inflammatory bowel disease,IBD)患者结直肠癌发病率高于正常人群[7]。
间充质干细胞(mesenchymal stem cells,MSCs)是来源于中胚层的一类成体干细胞,具有易于分离培养、免疫源性低及多向分化潜能等特性,同时还具有向肿瘤局部特异性趋化的特性,目前已有研究表明MSCs可以被募集至实质性肿瘤部位[8, 9, 10]。肿瘤的生长、侵袭和转移是肿瘤细胞与周围微环境及机体内环境之间相互作用的结果。作为肿瘤微环境的一部分,MSCs具有类似“免疫豁免”效应,可对各主要类型的免疫细胞产生增殖和活化抑制作用[11, 12, 13, 14],并通过低免疫表型、分泌抗炎分子等多种机制形成一个“免疫抑制微环境”,有利于肿瘤生长和逃逸。
在结肠癌的治疗中,化疗抵抗导致的化疗失败使得结肠癌的化疗效果有待进一步改进[15]。MSCs在炎症微环境下可通过免疫抑制和血管生成促进肿瘤的生长[9],但在肿瘤生长的炎症微环境中,MSCs在结肠癌细胞化疗抵抗中的作用尚不明确,本研究将探讨炎症微环境中MSCs对结肠癌细胞化疗抵抗的影响及其可能的机制。
1 材料和方法 1.1 材料人结肠癌细胞株HCT116细胞、HT29细胞及人骨髓MSCs均为第二军医大学东方肝胆外科医院肿瘤免疫与基因治疗实验室保存。流式细胞仪Aria购自美国BD公司,McCoy’s 5A培养液、胎牛血清(FBS)、胰酶购自美国Gibco公司,炎症因子干扰素γ(IFN-γ)、肿瘤坏死因子α(TNF-α)购自英国PeproTech公司,顺铂(批号:H37021357)购自齐鲁制药有限公司,5-氟尿嘧啶(5-FU,批号:D1308009)购自Sigma公司,Annexin Ⅴ-FITC/PI试剂盒购自南京凯基生物科技发展有限公司。
1.2 细胞培养及处理HCT116、HT29、MSCs分别采用McCoy’s 5A培养液、RPMI1640培养液和DMEM/F12培养液(10%FBS)培养,0.25%胰酶溶液消化后传代。在37℃、5%CO2培养箱条件下采用贴壁细胞培养法培养。取对数生长期的HCT116、HT29、MSCs,用含EDTA的0.25%胰酶溶液消化,收集细胞,用PBS洗涤、离心(1 000 r/min,5 min,r=16 cm)2次,备用。按2×104/孔的标准将MSCs细胞接种于6孔板中,每孔培养液加至2 mL,置于细胞培养箱中培养。MSCs单独培养或分别加炎症因子IFN-γ(20 ng/mL)、TNF-α(20 ng/mL)刺激12 h后换无血无抗培养液,继续培养24 h后收集上清备用。
1.3 药物浓度确定及实验分组 1.3.1 5-FU和顺铂浓度确定分别采用不同浓度的5-FU(0、0.025、2.5、25、100、200 μg/mL)和顺铂(0、1、5、20、50、100 μmol/L)处理HCT116细胞系和HT29细胞系,采用MTT方法检测细胞活性。当5-FU浓度为25 μg/mL、顺铂浓度为20 μmol/L时,对HCT116和HT29两种结肠癌细胞系的生长抑制率约为50%,因而本研究选择这两个浓度作为两种药物处理细胞的最佳浓度。
1.3.2 实验分组HCT116细胞系分为空白对照组、MSCs组、MSCs(I)组、MSCs(T)组、MSCs(IT)组、顺铂组、顺铂+MSCs组、顺铂+MSCs(I)组、顺铂+MSCs(T)组、顺铂+MSCs(IT)组。HT29细胞系分为空白对照组、MSCs组、MSCs(I)组、MSCs(T)组、MSCs(IT)组、5-FU组、5-FU+MSCs组、5-FU+MSCs(I)组、5-FU+MSCs(T)组、5-FU+MSCs(IT)组。空白对照组采用正常培养液培养;MSCs组采用未经处理的MSCs的条件培养上清培养;MSCs(I)组采用经IFN-γ处理MSCs后取得的条件培养上清培养;MSCs(T)组采用经TNF-α处理MSCs后取得的条件培养上清培养;MSCs(IT)组采用经IFN-γ和TNF-α共同处理MSCs后取得的条件培养上清培养。顺铂组、顺铂+MSCs组、顺铂+MSCs(I)组、顺铂+MSCs(T)组、顺铂+MSCs(IT)组和5-FU组、5-FU +MSCs组、5-FU+MSCs(I)组、5-FU+MSCs(T)组、5-FU+MSCs(IT)组分别在空白对照组、MSCs组、MSCs(I)组、MSCs(T)组、MSCs(IT)组的基础上同时进行顺铂或5-FU处理。作用时间为24 h,光镜下观察不同处理组HCT116细胞的形态学变化。
1.4 MTT法检测细胞增殖分别用McCoy’s 5A培养液(10%FBS)和RPMI1640培养液将HCT116细胞和HT29细胞配成单个细胞悬液,接种到96孔板(1×104/孔),每孔加液至200 μL。按实验分组每组3复孔,用药处理24 h后,去除培养液,每孔加入5 g/L的MTT溶液20 μL,继续孵育4 h,然后吸弃孔内培养上清液,每孔加150 μL DMSO,振荡10 min。待结晶物充分溶解,使用酶标仪在490 nm波长下测定各孔光密度(D)值。观察细胞的增殖能力。细胞生长增殖率(%)=(D对照组-D实验组)/D对照组×100%。
1.5 流式细胞仪检测细胞凋亡根据Annexin Ⅴ FITC/PI试剂盒使用说明,每组细胞消化离心(1 000 r/min,r=16 cm) 2次,弃上清,加入500 μL结合缓冲液和5 μL染料,避光20 min,上机检测。
1.6 RT-PCR检测凋亡相关基因Bax和Bcl-2 mRNA表达水平 用McCoy’s 5A培养液(10%FBS)将HCT116细胞配成单个细胞悬液,接种到6孔板(2×104孔/mL),12 h后按照实验分组分别进行药物处理,药物作用24 h后提取各组RNA。按照RT-PCR试剂盒说明书,对Bax和Bcl-2 mRNA的表达水平进行检测。Bcl-2引物序列:正义5′-CAG CTG CAC CTG ACG CCC TT-3′,反义5′-GCC TCC GTT ATC CTG GAT CC-3′; Bax引物序列:正义5′-TGC TTC AGG GTT TCA TCC AGG-3′,反义5′-TGG CAA AGT AGA AAA GGG CGA-3′。PCR反应条件:95℃预变性4 min;94℃ 30 s、56℃ 30 s、72℃ 90 s,30个循环;72℃延伸7 min。PCR产物用2%琼脂糖凝胶电泳进行鉴定。 1.7 统计学处理用SPSS 10.0软件进行统计学分析。所有实验数据均以 x±s 表示,所得数据经正态分析和方差齐性检验后,组间数据的差异比较采用单因素方差分析。检验水准(α)为0.05。
2 结 果 2.1 炎症因子刺激MSCs对HCT116细胞形态的影响在光镜下观察空白对照组、MSCs组、MSCs(I)组、MSCs(T)组、MSCs(IT)组和顺铂组、顺铂+MSCs组、顺铂+MSCs(I)组、顺铂+MSCs(T)组、顺铂+MSCs(IT)组的HCT116细胞形态,结果如图 1所示。可见未经顺铂处理的HCT116细胞形态正常;顺铂处理组细胞,细胞形态学发生改变,细胞皱缩且漂浮死细胞增多,但顺铂+MSCs(IT)组细胞形态接近正常。

|
图 1 光镜下不同处理组HCT116细胞形态学改变 Fig 1 Effects of different treatments on the morphology of HCT116 cells under light microscopy Control: Normal medium; MSCs: The supernatant of mesenchymal stem cells (MSCs); MSCs(I): The supernatant of MSCs treated by interferon-γ (IFN-γ); MSCs (T): The supernatant of MSCs treated by tumor necrosis factor-α (TNF-α); MSCs (IT): The supernatant of MSCs treated by IFN-γ and TNF-α. Cell morphology was observed and photographed by phase contrast microscopy. Original magnification: ×400 |
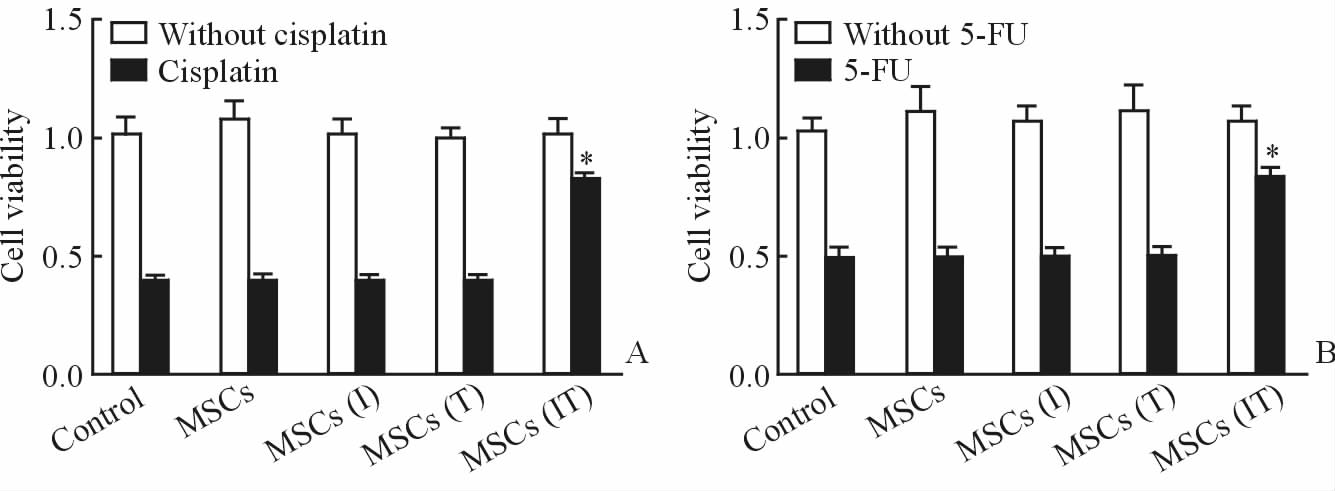
采用MTT方法对不同分组的HCT116细胞系和HT29细胞系的细胞活性进行检测,结果如图 2所示。可见未经顺铂处理的HCT116细胞的增殖能力均较好;经过顺铂处理过的细胞,除顺铂+MSCs(IT)组外,其他组的细胞增殖能力明显下降。未经5-FU处理的HT29细胞的增殖能力均较好;经过5-FU处理过的HT29细胞,除5-FU+MSCs(IT)组外,其他组的细胞增殖能力明显下降。
|
图 2 不同处理组HCT116(A)和HT29(B)细胞的增殖能力 Fig 2 Cell viability of HCT116 (A) and HT29 (B) cells receiving different treatments Control: Normal medium; MSCs: The supernatant of mesenchymal stem cells (MSCs); MSCs(I): The supernatant of MSCs treated by interferon-γ (IFN-γ); MSCs(T): The supernatant of MSCs treated by tumor necrosis factor-α (TNF-α); MSCs(IT): The supernatant of MSCs treated by IFN-γ and TNF-α. 5-FU: 5-Fluorouracil. *P<0.05 vs without cisplatin or 5-FU. n=3, x±s |
采用流式细胞仪检测不同处理组HCT116细胞和HT29细胞的凋亡情况,结果如表 1所示。可见HCT116细胞系对照组细胞无明显细胞凋亡;经顺铂处理的各组细胞凋亡明显,但顺铂+MSCs(IT)组HCT116细胞未发生明显凋亡,而且顺铂+MSCs(I)组和顺铂+MSCs(T)组细胞凋亡差异无统计学意义。HT29细胞系对照组细胞无明显细胞凋亡;经5-FU处理的各组细胞凋亡明显,但5-FU+MSCs(IT)组细胞未发生明显凋亡,而且5-FU+MSCs(I)组和5-FU+MSCs(T)组细胞凋亡差异无统计学意义。
|
|
表 1 不同处理组HCT116和HT29细胞的凋亡情况 Tab 1 Effects of different treatments on apoptosis of HCT116 and HT29 cells |
RT-PCR检测结果如图 3所示,与对照组比较,MSC(IT)条件培养上清可诱导HCT116细胞中Bcl-2 mRNA表达增加,Bax mRNA表达减少。

|
图 3 不同处理组HCT116细胞Bax和Bcl-2 mRNA的表达情况 Fig 3 Effects of different treatments on mRNA levels of Bax and Bcl-2 in HCT116 cells 1-5 represent HCT116 cells pretreated by normal medium, the supernatant of mesenchymal stem cells (MSCs), the supernatant of MSCs treated by interferon-γ(IFN-γ), the supernatant of MSCs treated by tumor necrosis factor-α(TNF-α), and the supernatant of MSCs treated by IFN-γ combined with TNF-α, respectively |
MSCs是来源于中胚层的具有多向分化潜能的干细胞,主要存在于骨髓组织和结缔组织间质中。在一定的诱导条件下,MSCs既可以向成骨细胞、脂肪细胞等中胚层细胞分化[16, 17],也可以向外胚层的神经细胞及内胚层的肝卵圆细胞分化[18, 19, 20]。MSCs不但免疫原性很弱,而且还具有免疫抑制作用,其特殊的免疫学特性越来越引起学者们的关注。本研究发现,经炎症因子IFN-γ和TNF-α联合预处理后的MSCs条件培养上清较未经炎症因子处理组增强结肠癌细胞化疗抵抗能力,化疗药物在上述条件培养上清中抑制结肠癌细胞增殖、促进结肠癌细胞凋亡的能力降低,提示炎症因子可通过诱导MSCs分泌可溶性因子增强结肠癌细胞的化疗抵抗能力。
Bcl-2被认为是细胞凋亡调控的最后通路之一。Bcl-2属于跨膜蛋白,主要分布于核膜、线粒体膜和内质网膜上,Bcl-2高表达的细胞能耐受多种细胞毒性因子的杀伤作用,使细胞凋亡明显延迟、减少,并且在毒性因子去除后可使细胞迅速恢复增生。Bcl-2编码的产物Bcl-2蛋白可通过拮抗野生型p53蛋白的促凋亡作用,将c-myc蛋白由促凋亡逆转为抑制凋亡,稳定线粒体的结构和功能及阻断Caspase酶类活化的上游信号传递通路等作用,抑制多种因素诱发的细胞凋亡,而Bax作为Bcl-2的同源体,其作用是抑制Bcl-2作用,其与Bcl-2一样在组织广泛表达。Bax与Bcl-2在细胞中的比例决定细胞是否发生凋亡。Bax过量时,形成Bax-Bax同二聚体,诱导细胞凋亡;Bcl-2过量时,形成Bcl-2-Bax异二聚体,抑制细胞凋亡[21]。本研究中我们发现,经过IFN-γ和TNF-α预处理后的MSCs条件培养上清可诱导结肠癌细胞Bcl-2基因表达上调,Bax基因表达下调,提示炎症微环境中MSCs增强结肠癌细胞化疗抵抗能力可能与其调控Bax和Bcl-2基因的表达有关。
MSCs具有向肿瘤组织中特异性趋化的特性,并在肿瘤的发生发展中起到重要作用。Djouad等[22]在免疫功能正常的小鼠皮下植入异基因B16黑素瘤细胞,在MSCs存在的情况下其增殖形成肿瘤。而在正常情况下,这些异基因肿瘤细胞会被受者免疫系统排斥。他们还发现不论与肿瘤细胞共同接种、系统性输注还是在远离肿瘤处皮下注射MSCs均可引起肿瘤形成。与上述结果相反,Khakoo等[23]在Kaposi’s 肉瘤小鼠模型中观察到,静脉输注的人骨髓MSCs可聚集到肿瘤处,并以剂量依赖的方式抑制肿瘤生长。研究还发现,MSCs在体内的抑瘤作用与其抑制靶细胞Akt蛋白激酶活性有关。此外,MSCs可以作为药物载体运输抗肿瘤药物到恶性肿瘤内部,Komarova等[24]把转染人工受体的MSC与肿瘤标记捆绑在一起,这种转染人工受体的MSCs具有更好的肿瘤趋向性,从而增加肿瘤靶向给药的功效。上述研究提示,MSCs可能是肿瘤微环境的重要组成部分,但MSCs在肿瘤微环境中对肿瘤发生发展及转归的作用及其内在机制仍需进一步深入研究。
| [1] | Arzumanyan A, Reis H M, Feitelson M A. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma[J]. Nat Rev Cancer, 2013, 13: 123-135. |
| [2] | Jing Y Y, Liu W T, Guo S W, Ye F, Fan Q M, Yu G F, et al. Hepatitis B virus (HBV) receptors: deficiency in tumor results in scant HBV infection and overexpression in peritumor leads to higher recurrence risk[J]. Oncotarget, 2015 Oct 20. doi: 10.18632/oncotarget.5518. [Epub ahead of print] |
| [3] | Haq N, Khan B A, Imran M, Akram A, Jamal A B, Bangash F. Frequency of gall bladder carcinoma in patients with acute and chronic cholecystitis[J]. J Ayub Med Coll Abbottabad, 2014, 26: 191-193. |
| [4] | Heemskerk J, Nienhuijs S W. Gallstone ileus as first presentation of a gallbladder carcinoma[J]. J Gastrointestin Liver Dis, 2009, 18: 253-254. |
| [5] | Bessède E, Dubus P, Mégraud F, Varon C. Helicobacter pylori infection and stem cells at the origin of gastric cancer[J]. Oncogene, 2015, 34: 2547-2555. |
| [6] | Graham D Y. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer[J]. World J Gastroenterol, 2014, 20: 5191-5204. |
| [7] | Collins P D. Strategies for detecting colon cancer and dysplasia in patients with inflammatory bowel disease[J]. Inflamm Bowel Dis, 2013, 19: 860-863. |
| [8] | Gao P, Ding Q, Wu Z, Jiang H, Fang Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma[J]. Cancer Lett, 2010, 290: 157-166. |
| [9] | Bergfeld S A, DeClerck Y A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment[J]. Cancer Metastasis Rev, 2010, 29: 249-261. |
| [10] | Gao Z, Zhang L, Hu J, Sun Y. Mesenchymal stem cells: a potential targeted-delivery vehicle for anti-cancer drug, loaded nanoparticles[J]. Nanomedicine, 2013, 9: 174-184. |
| [11] | Li M, Sun X, Kuang X, Liao Y, Li H, Luo D. Mesenchymal stem cells suppress CD8+T cell-mediated activation by suppressing natural killer group 2, member D protein receptor expression and secretion of prostaglandin E2, indoleamine 2, 3-dioxygenase and transforming growth factor-beta[J]. Clin Exp Immunol, 2014, 178: 516-524. |
| [12] | Zheng G, Ge M, Qiu G, Shu Q, Xu J. Mesenchymal stromal cells affect disease outcomes via macrophage polarization[J]. Stem Cells Int, 2015,2015: 989473. |
| [13] | Alikarami F, Yari F, Amirizadeh N, Nikougoftar M, Jalili M A. The Immunosuppressive activity of amniotic membrane mesenchymal stem cells on T lymphocytes[J]. Avicenna J Med Biotechnol, 2015, 7: 90-96. |
| [14] | De Miguel M P, Fuentes-Julián S, Blázquez-Martínez A, Pascual C Y, Aller M A, Arias J, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications[J]. Curr Mol Med, 2012, 12: 574-591. |
| [15] | Vellinga T T, Borovski T, de Boer V C, Fatrai S, van Schelven S, Trumpi K, et al. SIRT1/PGC1alpha-dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer[J]. Clin Cancer Res, 2015, 21: 2870-2879. |
| [16] | Granero-Molto F, Weis J A, Longobardi L, Spagnoli A.Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther,2008, 8: 255-268. |
| [17] | Vashi A V, Keramidaris E, Abberton K M, Morrison W A, Wilson J L, O'Connor A J, et al. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro[J]. Biomaterials,2008,29:573-579. |
| [18] | Pacary E, Tixier E, Coulet F, Roussel S, Petit E, Bernaudin M. Crosstalk between HIF-1 and ROCK pathways in neuronal differentiation of mesenchymal stem cells, neurospheres and in PC12 neurite outgrowth[J]. Mol Cell Neurosci,2007,35: 409-423. |
| [19] | Porada C D, Zanjani E D, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications[J]. Curr Stem Cell Res Ther,2006,1: 365-369. |
| [20] | Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G,et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes[J]. Hepatology,2007, 46: 219-228. |
| [21] | Samarghandian S, Nezhad M A, Mohammadi G. Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells[J]. Anticancer Agents Med Chem,2014, 14: 901-909. |
| [22] | Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J,et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals[J]. Blood,2003, 102: 3837-3844. |
| [23] | Khakoo A Y, Pati S, Anderson S A, Reid W, Elshal M F, Rovira I I,et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma[J]. J Exp Med, 2006,203:1235-1247. |
| [24] | Komarova S, Roth J, Alvarez R, Curiel D T, Pereboeva L.Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor[J]. J Ovarian Res,2010, 3: 12. |
 2016, Vol. 37
2016, Vol. 37


