腋窝淋巴结(axillary lymph node,ALN)状况是预测乳腺癌患者预后的主要影响因素。2015年美国国家综合癌症网络(NCCN)指南提倡使用前哨淋巴结活检术(sentinel lymph node biopsy,SLNB)替代腋窝淋巴结清扫术成为腋窝淋巴结预测的手段[1]。对于SLNB结果阳性的患者需要进一步行腋窝淋巴结清扫术,而结果阴性的患者则不需要。对于SLNB明确前哨淋巴结肿瘤转移而未行腋窝淋巴结清扫术的部分患者,腋窝的局部复发风险仍然低于1%[2]。ACOSOG Z0011的研究结果也同样证实了这一点[3]。
SLNB最初应用于早期乳腺癌患者。近年来聚焦于新辅助化疗(neoadjuvant chemotherapy,NCT)实施前后SLNB的研究越来越多,但SLNB在行NCT的乳腺癌患者中如何应用仍然存在争议。NCT后行SLNB是否能准确反映腋窝淋巴结的状况是我们关注的焦点。本研究汇总了对NCT后乳腺癌患者实施SLNB的文献,以整合计算NCT后SLNB的检出率及假阴性率,判断其否可应用于临床。
1 材料和方法 1.1 文献检索中文文献以“乳腺癌”、“新辅助化疗”及“前哨淋巴结活检”为自由词在中国生物医学文摘数据库(CBM)上进行检索;非中文文献以“breast cancer”、“neoadjuvant chemotherapy”和“sentinel lymph node biopsy”为自由词和主题词在PubMed、Medline及Embase上进行检索。检索文献日期由1990年1月1日至2014年4月1日。
1.2 文献纳入标准(1)研究对象均为经组织学证实的浸润性乳腺癌患者;(2)NCT后腋窝淋巴结阴性;(3)NCT后行SLNB,无论前哨淋巴结状况如何,均随后实施腋窝淋巴结清扫术;(4)明确记录NCT前腋窝淋巴结状况;(5)根据石蜡切片进行腋窝淋巴结的肿瘤转移诊断;(6)能够提取明确的研究例数、检出率、假阴性例数、假阴性率;(7)同一研究机构或研究者发表的多项研究,选择最近更新的研究。将临床查体、影像学检查(B超、钼靶、MRI)及细针穿刺细胞学检查提示腋窝淋巴结存在肿瘤转移定义为腋窝淋巴结阳性。
1.3 文献排除标准(1)仅接受新辅助内分泌治疗者;(2)摘要中无法获得所需数据的非中英文文献;(3)研究对象为炎性乳癌者。
1.4 数据提取根据NCT前腋窝淋巴结状况分为腋窝淋巴结阴性组和阳性组,分别对文献数据进行整理归纳。记录纳入文献的发表时间、病例总数、检出前哨淋巴结例数和检出率、假阴性例数、腋窝淋巴结阳性例数及假阴性率。检出率=(前哨淋巴结检出例数/实施SLNB的所有例数)×100%;假阴性率=(前哨淋巴结假阴性例数/腋窝淋巴结转移阳性例数)×100%。前哨淋巴结为阴性而腋窝淋巴结为阳性的病例定义为前哨淋巴结假阴性。
1.5 统计学处理采用STATA 11.0软件进行统计学分析。使用随机效应模型计算前哨淋巴结检出率、假阴性率及95%可信区间(CI)。检验水准(α)为0.05。
2 结 果 2.1 纳入文献基本情况文献筛选流程图如图 1所示。本研究共纳入41篇原创性研究文献,发表时间自2003年至2014年,共计5 848例患者,分为NCT前腋窝淋巴结阴性组和阳性组2组,各组例数分别为2 050、3 798例。
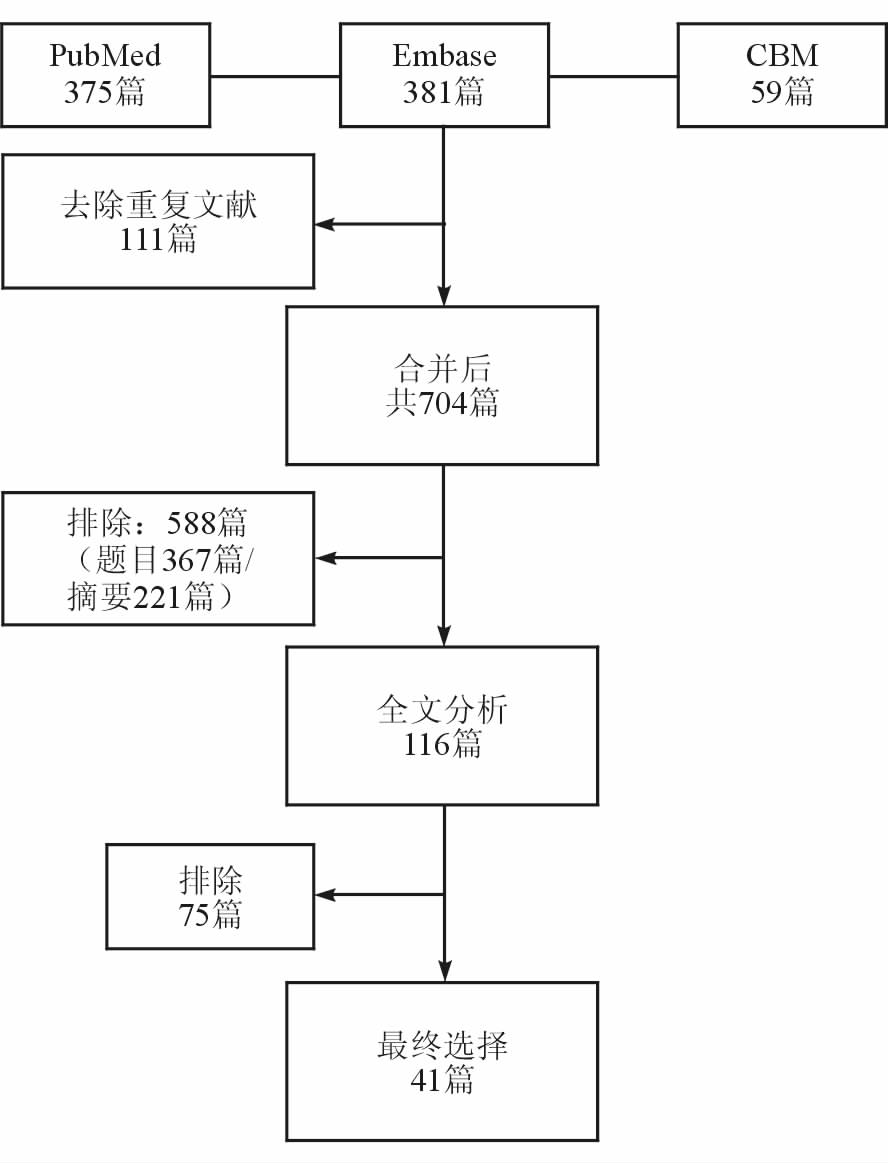
|
图 1 文献筛选流程图 |
NCT前腋窝淋巴结阴性组共纳入24项原创性研究[4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27],纳入研究的发表时间自2003年至2014年,共计2 050例患者,其中1 891例患者成功检出前哨淋巴结(文献[24]无检出前哨淋巴结例数,予以排除)。将上述研究结果进行单个样本率的meta分析后得到阴性组患者NCT后行SLNB的检出率为0.94(95%CI 0.92~0.96),见图 2。假阴性例数64例,腋窝淋巴结阳性病例606例,假阴性率为0.07(95%CI 0.04~0.10),见图 3(文献[7]、[16]和[19]无假阴性例数,予以排除)。为排除因纳入病例数较少而造成的统计偏移,研究又分为病例数≥50例和病例数<50例2个亚组,两组的前哨淋巴结检出率分别为0.93(95%CI 0.89~0.97)和0.97(95%CI 0.95~0.99),假阴性率分别为0.09(95%CI 0.04~0.13)和0.06(95%CI 0.01~0.10),差异无统计学意义。
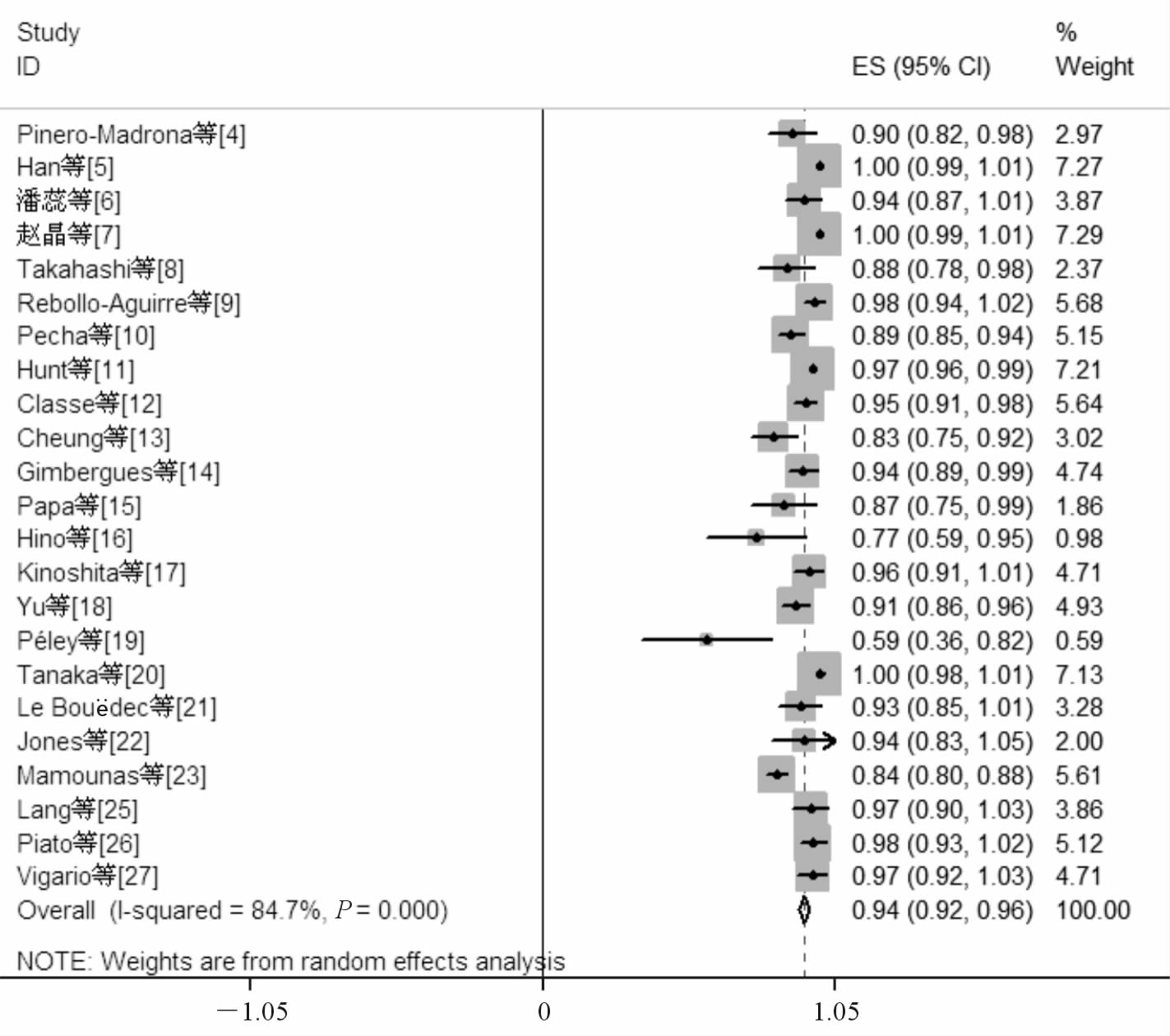
|
图 2 新辅助化疗(NCT)前腋窝淋巴结阴性组前哨淋巴结检出率 |
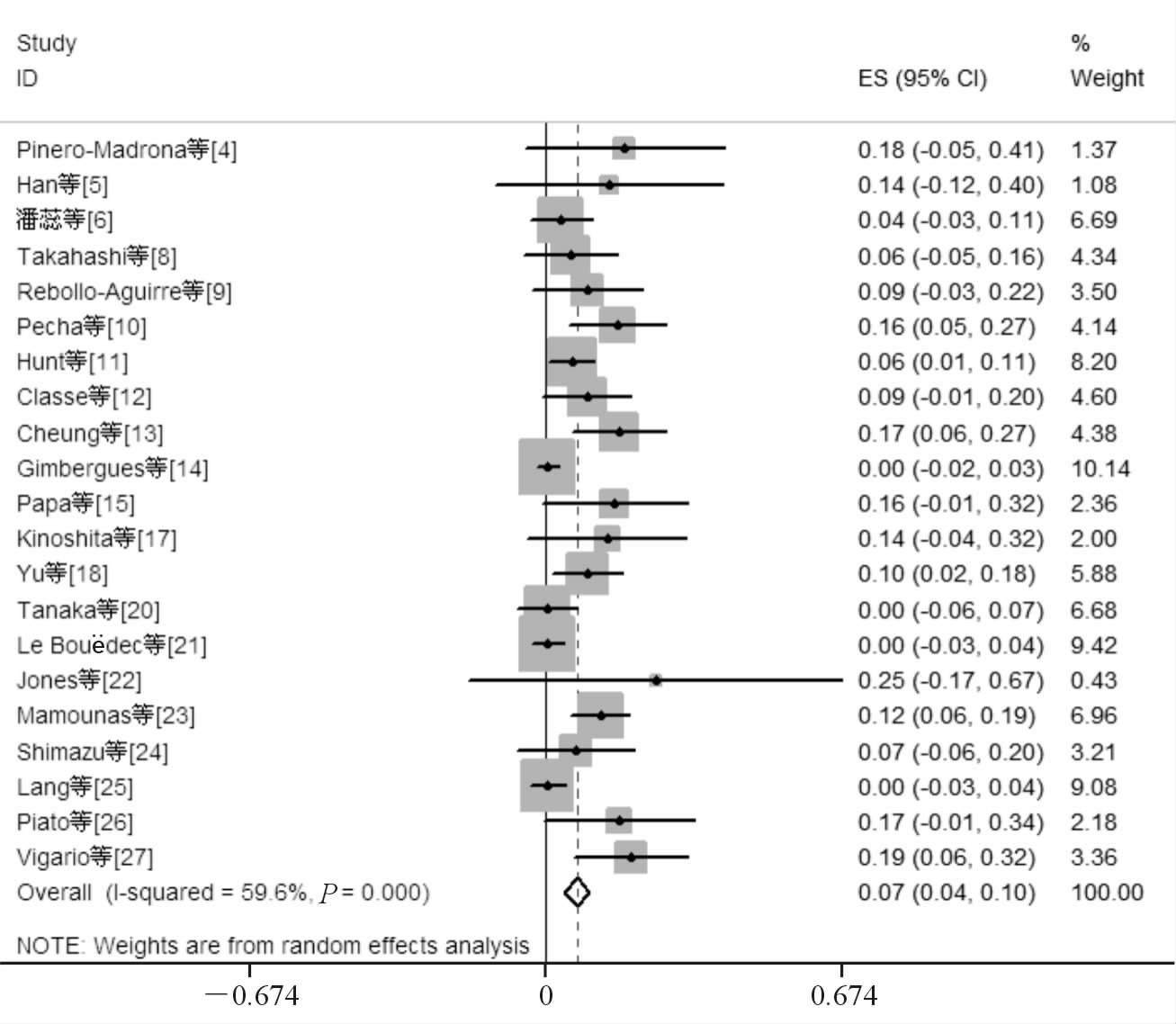
|
图 3 新辅助化疗(NCT)前腋窝淋巴结阴性组假阴性率 |
NCT前腋窝淋巴结阳性组共纳入32项原创性研究[4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 20, 21, 22, 23, 25, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44],纳入研究的发表时间自2004年至2014年,共计3 798例患者,其中3 313例患者成功检出前哨淋巴结。将上述研究结果进行单个样本率的meta分析后得到阳性组患者NCT后行SLNB的检出率为0.87(95%CI 0.84~0.90),见图 4(文献[44]无检出前哨淋巴结例数,予以排除)。假阴性例数266例,腋窝淋巴结阳性病例1 843例,假阴性率为0.13(95%CI 0.11~0.16),见图 5(文献[7]和[16]无假阴性例数,予以排除)。为排除因纳入病例数较少的研究造成的统计偏移,研究分为病例数≥50例和病例数<50例2个亚组,两组的前哨淋巴结检出率分别为0.87(95%CI 0.84~0.90)和0.89(95%CI 0.83~0.95),假阴性率分别为0.13(95%CI 0.10~0.16)及0.16(95%CI 0.07~0.23),差异无统计学意义。

|
图 4 新辅助化疗(NCT)前腋窝淋巴结阳性组前哨淋巴结检出率 |

|
图 5 新辅助化疗(NCT)前腋窝淋巴结阳性组假阴性率 |
本研究结果发现NCT前腋窝淋巴结阳性的乳腺癌患者在NCT后行SLNB的前哨淋巴结检出率为0.87,低于阴性患者(0.94),原因可能是阳性患者的淋巴管内存在肿瘤细胞,这部分肿瘤细胞被杀死后堵塞淋巴管,导致淋巴管显影剂的回流受阻,从而不能使淋巴管和前哨淋巴结达到理想显影,最终导致了检出率的下降。
SLNB的假阴性率是一个十分重要的指标,假阴性率的高低决定着SLNB的准确程度及其是否能够应用于临床。本研究发现NCT前腋窝淋巴结阳性的患者在NCT后行SLNB的假阴性率为0.13,未达到2015年NCCN指南中实施SLNB需要满足假阴性率低于10%的准入标准[1];而NCT前腋窝淋巴结阴性患者的假阴性率为0.07,完全达到准入标准,两者几乎相差1倍。阳性患者假阴性率的升高可能与腋窝淋巴结在NCT后的缓解顺序有关,NCT前前哨淋巴结与非前哨淋巴结均存在肿瘤转移的患者,在NCT结束后,前哨淋巴结已发生病理学完全缓解而非前哨淋巴结尚未发生病理学完全缓解。在这种情况下,SLNB的结果不能真实的反映腋窝淋巴结状况,从而导致假阴性结果的产生。
王永胜等[45]研究发现,SLNB完成40例以上才有资质应用于临床。考虑到病例数量可能对结果产生影响,本研究对纳入文献以50例为标准进行了分组,目的是排除因SLNB技术不熟练导致的检出率下降及假阴性率升高。结果显示,无论对于NCT前腋窝淋巴结阴性还是阳性的患者,纳入病例数多少对于前哨淋巴结检出率及假阴性率均影响不大,原因可能是纳入文献的研究者在进行研究前均已完成了相当数量的SLNB训练,所以样本量的大小不会对手术操作水平产生较大影响。
Boughey等[29]的研究提示对于检出3个以上前哨淋巴结的乳腺癌患者,其假阴性率会较检出2个及以下前哨淋巴结的患者显著下降(9.1% vs 21.1%)。Krag等[46]研究发现,对于化疗前行SLNB的乳腺癌患者,随着前哨淋巴结检出数量的增加,假阴性率不断下降:前哨淋巴结检出数量为1个的假阴性率为18%,2个时假阴性率为10%,3个时假阴性率为7%。与之相同,Hunt等[11]研究发现,对于临床上腋窝淋巴结阴性的患者,NCT后行SLNB,前哨淋巴结检出数量为2个及以下的病例组假阴性率更高。本研究中可以根据前哨淋巴结检出个数进行分层的文献数量很少,所以未对此进行进一步研究。
本研究尚存在以下局限性:文献来源主要为中英文文献,其余语言文献仅少量纳入;数据录入存在选择偏移。此外,本研究结果的异质性较高,可能与临床异质性和方法学异质性相关。临床异质性主要有两个方面,一是纳入文献对于腋窝淋巴结阳性的判定标准不尽一致,包括了临床体检、影像学判定及病理性判定(细针穿刺及B超引导下细针穿刺),前两者的判定不一定明确对应腋窝淋巴结存在肿瘤转移,腋窝淋巴结在NCT后的缓解顺序假说支持证据不够充分;二是纳入文献中SLNB所使用的示踪方法不同,有单用染料、单用核素以及两者合用等方法,示踪方法的异质性可能导致结果的异质性。方法学异质性主要与前哨淋巴结的阳性判定有关,孤立肿瘤细胞阳性是否被定义为前哨淋巴结阳性一直存在争议,纳入文献中约一半未对此问题进行详细说明。
综上所述,NCT前腋窝淋巴结阴性的乳腺癌患者可以在NCT后行SLNB,但NCT前腋窝淋巴结阳性的患者NCT后行SLNB的检出率较低,而假阴性率较高,所以不建议NCT后行SLNB。
| [1] | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer(version.2.2015) [EB/OL]. (2015)[2015-07-29]. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf |
| [2] | Spiguel L, Yao K, Winchester D J, Gorchow A, Du H, Sener S F, et al. Sentinel node biopsy alone for node-positive breast cancer: 12-year experience at a single institution[J]. J Am Coll Surg, 2011, 213: 122-128. |
| [3] | Caudle A S, Hunt K K, Kuerer H M, Meric-Bernstam F, Lucci A, Bedrosian I, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial[J]. Ann Surg Oncol,2011,18:2407-2412. |
| [4] | Pinero-Madrona A, Escudero-Barea M J, Fernandez-Robayna F, Alberro-Adúriz J A, García-Fernández A, Vicente-García F, et al. Selective sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer: results of the GEICAM 2005-07 study[J]. Cir Esp,2015,93: 23-29. |
| [5] | Han A, Moon H G, Kim J, Ahn S K, Park I A, Han W, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients[J]. J Breast Cancer, 2013, 16: 378-385. |
| [6] | 潘 蕊,杨 奔,左文述,杨 莉,王永胜,郑 刚,等. 乳腺癌新辅助化疗后前哨淋巴结活检临床应用价值的探讨[J].中华肿瘤防治杂志,2012,19: 1883-1888. |
| [7] | 赵 晶,宋志武,黄 焰,郝晓鹏,梁 峰,王世彬,等. 乳腺癌围新辅助化疗期前哨淋巴结活检的可行性分析[J].中华医学杂志,2012,92: 2538-2541. |
| [8] | Takahashi M, Jinno H, Hayashida T, Sakata M, Asakura K, Kitagawa Y. Correlation between clinical nodal status and sentinel lymph node biopsy false negative rate after neoadjuvant chemotherapy[J]. World J Surg, 2012, 36: 2847-2852. |
| [9] | Rebollo-Aguirre A C, Gallego-Peinado M, Menjon-Beltran S, García-García J, Pastor-Pons E, Chamorro-Santos C E, et al. Sentinel lymph node biopsy in patients with operable breast cancer treated with neoadjuvant chemotherapy[J]. Rev Esp Med Nucl Imagen Mol,2012,31: 117-123. |
| [10] | Pecha V, Kolarik D, Kozevnikova R, Hovorkova K, Hrabetova P, Halaska M, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy[J]. Cancer, 2011, 117: 4606-4616. |
| [11] | Hunt K K, Yi M, Mittendorf E A, Guerrero C, Babiera G V, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients[J]. Ann Surg, 2009, 250: 558-566. |
| [12] | Classe J M, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study[J]. J Clin Oncol, 2009, 27: 726-732. |
| [13] | Cheung T T, Suen D T, Kwong A. Is sentinel lymph node biopsy after neoadjuvant chemotherapy feasible in Chinese patients with invasive breast cancers [J].ANZ J Surg, 2009, 79: 719-723. |
| [14] | Gimbergues P, Abrial C, Durando X, Le Bouedec G, Cachin F, Penault-Llorca F, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation[J]. Ann Surg Oncol, 2008, 15: 1316-1321. |
| [15] | Papa M Z, Zippel D, Kaufman B, Shimon-Paluch S, Yosepovich A, Oberman B, et al. Timing of sentinel lymph node biopsy in patients receiving neoadjuvant chemotherapy for breast cancer[J]. J Surg Oncol, 2008, 98: 403-406. |
| [16] | Hino M, Sano M, Sato N, Homma K. Sentinel lymph node biopsy after neoadjuvant chemotherapy in a patient with operable breast cancer[J]. Surg Today, 2008, 38: 585-591. |
| [17] | Kinoshita T. Sentinel lymph node biopsy is feasible for breast cancer patients after neoadjuvant chemotherapy[J]. Breast Cancer, 2007, 14: 10-15. |
| [18] | Yu J C, Hsu G C, Hsieh C B, Yu C P, Chao T Y. Role of sentinel lymphadenectomy combined with intraoperative ultrasound in the assessment of locally advanced breast cancer after neoadjuvant chemotherapy[J]. Ann Surg Oncol, 2007, 14: 174-180. |
| [19] | Péley G, Török K, Farkas E, Mátrai Z, Horváth Z, Sinkovics I, et al. [The feasibility and the role of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer] [J]. Magy Onkol, 2006, 50: 19-23. |
| [20] | Tanaka Y, Maeda H, Ogawa Y, Nishioka A, Itoh S, Kubota K, et al. Sentinel node biopsy in breast cancer patients treated with neoadjuvant chemotherapy[J]. Oncol Rep, 2006, 15: 927-931. |
| [21] | Le Bouëdec G, Geissler B, Gimbergues P, Cachin F, Penault-Llorca F, Kwiatkowski F, et al. [Sentinel lymph node biopsy for breast cancer after neoadjuvant chemotherapy: influence of nodal status before treatment] [J]. Bull Cancer, 2006, 93: 415-419. |
| [22] | Jones J L, Zabicki K, Christian R L, Gadd M A, Hughes K S, Lesnikoski B A, et al. A comparison of sentinel node biopsy before and after neoadjuvant chemotherapy: timing is important[J]. Am J Surg, 2005, 190: 517-520. |
| [23] | Mamounas E P, Brown A, Anderson S, Smith R, Julian T, Miller B, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27[J]. J Clin Oncol, 2005, 23: 2694-2702. |
| [24] | Shimazu K, Tamaki Y, Taguchi T, Akazawa K, Inoue T, Noguchi S. Sentinel lymph node biopsy using periareolar injection of radiocolloid for patients with neoadjuvant chemotherapy-treated breast carcinoma[J]. Cancer, 2004, 100: 2555-2561. |
| [25] | Lang J E, Esserman L J, Ewing C A, Rugo H S, Lane K T, Leong S P, et al. Accuracy of selective sentinel lymphadenectomy after neoadjuvant chemotherapy: effect of clinical node status at presentation[J]. J Am Coll Surg, 2004, 199: 856-862. |
| [26] | Piato J R, Barros A C, Pincerato K M, Sampaio A P, Pinotti J A. Sentinel lymph node biopsy in breast cancer after neoadjuvant chemotherapy. A pilot study[J]. Eur J Surg Oncol, 2003, 29: 118-120. |
| [27] | Vigario A, Sapienza M T, Sampaio A P, Piato J R, Barros N, Barros A, et al. Primary chemotherapy effect in sentinel node detection in breast cancer[J]. Clin Nucl Med, 2003, 28: 553-557. |
| [28] | 刘 广,邱鹏飞,王永胜,周正波,李永清,刘雁冰,等. 新辅助化疗后腋窝淋巴结转阴乳腺癌患者前哨淋巴结活检研究[J].中华内分泌外科杂志,2013,7: 111-114. |
| [29] | Boughey J C, Suman V J, Mittendorf E A, Ahrendt G M, Wilke L G, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial[J]. JAMA, 2013, 310: 1455-1461. |
| [30] | Park S, Park J M, Cho J H, Park H S, Kim S I, Park B W. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis[J]. Ann Surg Oncol, 2013, 20: 2858-2865. |
| [31] | Yagata H, Yamauchi H, Tsugawa K, Hayashi N, Yoshida A, Kajiura Y, et al. Sentinel node biopsy after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer[J]. Clin Breast Cancer, 2013, 13: 471-477. |
| [32] | Rebollo-Aguirre A C, Gallego-Peinado M, Sanchez-Sanchez R, Pastor-Pons E, García-García J, Chamorro-Santos C E, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with operable breast cancer and positive axillary nodes at initial diagnosis[J]. Rev Esp Med Nucl Imagen Mol, 2013, 32: 240-245. |
| [33] | Takei H, Yoshida T, Kurosumi M, Inoue K, Matsumoto H, Hayashi Y, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy predicts pathological axillary lymph node status in breast cancer patients with clinically positive axillary lymph nodes at presentation[J]. Int J Clin Oncol, 2013, 18: 547-553. |
| [34] | Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study[J]. Lancet Oncol, 2013, 14: 609-618. |
| [35] | Alvarado R, Yi M, Le-Petross H, Gilcrease M, Mittendorf E A, Bedrosian I, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer[J]. Ann Surg Oncol, 2012, 19: 3177-3184. |
| [36] | Canavese G, Dozin B, Vecchio C, Tomei D, Villa G, Carli F,et al. Accuracy of sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with locally advanced breast cancer and clinically positive axillary nodes[J]. Eur J Surg Oncol, 2011,37:688-694. |
| [37] | Ozmen V, Unal E S, Muslumanoglu M E, Igci A, Canbay E, Ozcinar B, et al. Axillary sentinel node biopsy after neoadjuvant chemotherapy[J]. Eur J Surg Oncol, 2010, 36: 23-29. |
| [38] | Newman E A, Sabel M S, Nees A V, Schott A, Diehl K M, Cimmino V M, et al. Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation[J]. Ann Surg Oncol, 2007, 14: 2946-2952. |
| [39] | Lee S, Kim E Y, Kang S H, Kim S W, Kim S K, Kang K W, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients[J]. Breast Cancer Res Treat, 2007, 102: 283-288. |
| [40] | Shen J, Gilcrease M Z, Babiera G V, Ross M I, Meric-Bernstam F, Feig B W, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases[J]. Cancer, 2007, 109: 1255-1263. |
| [41] | Kinoshita T. Sentinel lymph node biopsy is feasible for breast cancer patients after neoadjuvant chemotherapy[J]. Breast Cancer, 2007, 14: 10-15. |
| [42] | Khan A, Sabel M S, Nees A, Diehl K M, Cimmino V M, Kleer C G, et al. Comprehensive axillary evaluation in neoadjuvant chemotherapy patients with ultrasonography and sentinel lymph node biopsy[J]. Ann Surg Oncol, 2005, 12: 697-704. |
| [43] | Kang S H, Kim S K, Kwon Y, Kang H S, Kang J H, Ro J, et al. Decreased identification rate of sentinel lymph node after neoadjuvant chemotherapy[J]. World J Surg, 2004, 28: 1019-1024. |
| [44] | Shimazu K, Tamaki Y, Taguchi T, Akazawa K, Inoue T, Noguchi S. Sentinel lymph node biopsy using periareolar injection of radiocolloid for patients with neoadjuvant chemotherapy-treated breast carcinoma[J]. Cancer, 2004, 100: 2555-2561. |
| [45] | 王永胜,欧阳涛,王启堂,苏逢锡,朱时光,吴 炅,等.中国前哨淋巴结活检多中心协作研究CBCSG-001最新资料报告[J].中华乳腺病杂志,2009,3:265-272. |
| [46] | Krag D N, Anderson S J, Julian T B, Brown A M, Harlow S P, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase Ⅲ trial[J]. Lancet Oncol, 2007, 8: 881-888. |
 2016, Vol. 37
2016, Vol. 37


