2. 第二军医大学长征医院消化内科, 上海 200003
2. Department of Gastroenterology, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China
肝细胞核因子4α(HNF4α)是核受体超家族成员之一,在肝细胞的分化和功能维持中起着重要的作用。我们前期研究表明,利用腺病毒载体过表达HNF4α可诱导肝癌细胞向成熟肝细胞分化,逆转肝细胞癌的恶性程度[1]。细胞穿膜肽(cell penetrating peptides,CPPs)是一类由30或少于30个氨基酸组成的多肽,可以有效地将目标分子包括蛋白、RNA、DNA等导入细胞。CPPs一般可分为两大类,一类是主要由赖氨酸阳离子残基组成、具有亲水和疏水氨基酸分布的两亲型;另一类是主要以精氨酸和赖氨酸残基为主的阳离子型[2]。
本研究利用原核表达载体表达并纯化了带有两亲型CPPs PEP-1(KET WWE TWW TEW SQP KKK RKV)的HNF4α 融合蛋白P-HNF4α,并研究其对肝癌细胞诱导分化的作用和肝癌细胞恶性表型的影响,探讨P-HNF4α对肝细胞癌的治疗潜能。
- 1 材料和方法 - 1.1 主要材料及试剂 原核表达载体pET28a购自Novagen公司,载体构建相关试剂购自TaKaRa公司。融合蛋白原核表达宿主BL21 (DE3) pLysS购自北京天根生化科技有限公司,异丙基硫代半乳糖苷(IPTG) 购自TaKaRa公司,咪唑购自Sigma公司,蛋白纯化Ni-NTA Agarose Beads购自Qiagen公司,其他蛋白纯化试剂购自国药集团化学试剂有限公司。人肝癌细胞株Huh7与Hep3B由第二军医大学长征医院消化内科实验室保存,细胞培养相关试剂均购自Gibco公司。核质分离试剂盒购自Invent Biotechnologies公司,RNA抽提试剂盒购自TaKaRa公司,CCK-8试剂盒购自Dojindo公司。Anti-HNF4α抗体和anti-Lamin A抗体购自Santa Cruz公司,anti-GAPDH抗体购自Sigma公司,anti-6×His抗体购自Abcam公司,IRDye标记的抗鼠、抗羊二抗购自LI-COR Biosciences公司。PCR引物均由Invitrogen公司合成。
1.2 细胞培养人肝癌细胞株Huh7用DMEM(含10%血清)、人肝癌细胞株Hep3B用MEM(含10%血清,1×非必需氨基酸)于37℃、5%CO2细胞培养箱中培养。
1.3 载体构建利用多重PCR首先将编码穿膜肽PEP-1(KET WWE TWW TEW SQP KKK RKV)的DNA片段与HNF4α编码片段插入pET28a质粒,构建了原核表达载体pET28a-P-HNF4α,该质粒可表达带有His标签氨基端连接PEP-1的HNF4α融合蛋白。引物序列如下:pET28a-PEP-1-HNF4α-F1,5′-GGA CCG AAT GGT CTC AGC CGA AAA AAA AAC GTA AAG TGC GAC TCT CCA AAA CCC TCG T-3′;pET28a-HNF4α-BamHⅠ-F2,5′-CGC GGA TCC AAA GAA ACC TGG TGG GAA ACC TGG TGG ACC GAA TGG TCT CAG CCG AAA-3′;pET28a-HNF4α-HindⅢ-R,5′-CCC AAG CTT CTA GAT AAC TTC CTG CTT GGT GAT GGT-3′),得到能够表达融合蛋白P-HNF4α的重组表达质粒pET28a-P-HNF4α。重组质粒经酶切鉴定后,送Invitrogen公司测序鉴定。
1.4 融合蛋白P-HNF4α的表达与纯化因载体pET28a多克隆位点上游存在一段表达六聚组氨酸(6×His)标签的序列,故诱导表达的融合蛋白6×His-PEP-1-HNF4α(P-HNF4α)可通过Ni-NTA Agarose Beads亲和层析纯化。pET28a-P-HNF4α质粒转化原核表达宿主菌大肠杆菌BL21,LB培养基37℃摇床培养过夜,次日以1∶50接种到800 mL LB培养基,37℃摇床培养。当D600达到1.0~1.2,分别加入终浓度为0、0.1、0.5、1.0 mmol/L的IPTG,17℃诱导表达16 h[3]或24℃诱导表达2 h。4℃ 6 000 r/min (离心半径为7 cm)收集菌体,于-80℃过夜。
用预冷裂解液[50 mmol/L NaH2PO4,300 mmol/L NaCl,10 mmol/L Imidazole,10%甘油(pH 8.0),100 μg/mL PMSF,3 mmol/L β-ME]重悬菌体。冰浴超声裂解细胞,4℃ 13 000 r/min (离心半径为7 cm) 离心收集上清。0.22 μm滤膜过滤上清,加入已用裂解液平衡的Ni-NTA Agarose Beads,4℃旋转结合2 h。用4倍柱体积洗涤液[50 mmol/L NaH2PO4,300 mmol/L NaCl,100 mmol/L Imidazole,10%甘油(pH 8.0)]洗3遍。最后用洗脱液[50 mmol/L NaH2PO4,300 mmol/L NaCl,500 mmol/L Imidazole,10%甘油(pH 8.0)]洗脱蛋白P-HNF4α。经过PEG20000浓缩和PBS(10%甘油)透析,所得蛋白用BCA蛋白定量试剂盒(碧云天)定量,SDS-PAGE蛋白电泳后按照快速银染法(碧云天)鉴定纯度。
1.5 P-HNF4α转染肝癌细胞以3×105个细胞/孔的细胞密度接种Huh 7细胞于6孔板,培养过夜。次日加入终浓度为25、50、100、200 nmol/L的P-HNF4α蛋白,同时做一组细胞不处理的CTR对照和10%甘油对照。48 h后收蛋白做蛋白质印迹。P-HNF4α一抗为anti-HNF4α,内参GAPDH一抗为anti-GAPDH。
1.6 蛋白质印迹检测用蛋白裂解液[100 mmol/L Tris-HCl (pH 6.8),4%SDS,20%甘油,200 mmol/L DTT]收集蛋白。常规8% SDS-PAGE凝胶电泳分离蛋白,转至硝酸纤维素膜。 5%脱脂奶粉/PBST (1×PBS,0.1% Tween20)封闭2 h,一抗于4℃湿盒孵育过夜。次日PBST洗涤3次,5 min/次;二抗室温湿盒避光孵育1 h,PBST避光洗涤3次,5 min/次;用Odyssey Infrared Imaging System(LI-COR Biosciences)在700~800 nm波长通道扫描。
1.7 观察指标 1.7.1 P-HNF4α穿膜效率蛋白质印迹检测上样量为0、0.1、0.2、0.3、0.4、0.5 μg的P-HNF4α,每个样品设两个复孔,扫描获得条带灰度值,利用相应的灰度值绘制标准曲线。以6×105个细胞/皿的细胞密度接种Huh7细胞于60 mm细胞培养皿,待细胞贴壁后,加入5.9 μg P-HNF4α至终浓度100 nmol/L,培养24 h后用50 μL蛋白裂解液收集蛋白,取5 μL细胞裂解液(约25 μg细胞总蛋白),蛋白质印迹检测转导至细胞内P-HNF4α的条带灰度值,根据标准曲线计算P-HNF4α的穿膜效率。
1.7.2 核质分离以6.5×105个细胞/皿的密度接种Huh7细胞于60 mm细胞培养皿,培养过夜。加P-HNF4α至终浓度100 nmol/L,设未处理的CTR对照以及10%甘油对照,培养48 h后,根据核质分离试剂盒MinuteTM Cytoplasmic and Nuclear Extraction Kit (Invent Biotechnologies)操作步骤做分离。核质抽提物用BCA蛋白定量试剂盒定量,蛋白质印迹检测核质抽提物中P-HNF4α蛋白量。P-HNF4α一抗为anti-HNF4α,胞质内参GAPDH一抗为anti-GAPDH,核内参Lamin A一抗为anti-Lamin A。
1.7.3 细胞免疫荧光以1×105个细胞/皿的密度接种Huh7细胞至带有包被PLL盖玻片的35 mm细胞培养皿中,培养过夜。加入终浓度为100 nmol/L的P-HNF4α融合蛋白。培养48 h后,细胞用预冷的PBS洗2遍,4%多聚甲醛室温固定20 min,0.1% Triton X-100打孔5 min,5%马血清室温封闭2 h,一抗anti-His(Abcam)4℃湿盒孵育过夜,ALEXA488荧光标记的Donkey anti-mouse二抗室温孵育30 min。细胞核用DAPI染色,荧光共聚焦显微镜扫描图像。
1.7.4 Real-PCR测定目的基因表达以1.2×105个细胞/孔的密度接种Huh7于12孔板细胞培养皿,培养过夜。加入终浓度为100 nmol/L的P-HNF4α融合蛋白,设10%甘油对照。培养48、72 h收细胞,按照RNAiso Plus(TaKaRa)法抽提样品总RNA,反转录试剂盒将RNA反转录成cDNA,设计real-time PCR引物(表 1),采用SYBR荧光定量法检测目的基因的表达。
|
|
表 1 Real-time PCR引物序列 Tab 1 Primers for real-time PCR |
以3 000个细胞/孔的密度接种Huh7和Hep3B细胞于96孔板细胞培养皿,培养过夜,次日换入含P-HNF4α的培养液,终浓度为0、25、100 nmol/L。CCK-8试剂盒(Dojindo)检测第0天到第6天细胞数。
1.7.6 细胞划痕实验将Huh7细胞以7×104细胞/孔的密度接种于24孔板,待融合度达70%时,使用无菌枪头在单层细胞上划痕,PBS清洗3次后换上含100 nmol/L P-HNF4α的DMEM培养液(10% FBS),设置加入相同体积10%甘油的对照和未处理对照,并在显微镜下拍照。于37℃细胞培养箱中培养,每间隔24 h拍照观察。
1.7.7 小室侵袭实验将Transwell小室上层用matrigel包被,后将Huh7细胞以7×104细胞/500 μL无血清DMEM混匀,加入终浓度为100 nmol/L的P-HNF4α(设置相同体积的PBS和10%甘油对照)。混匀后加入上室,下室加入1 mL含10% FBS的DMEM培养液。培养48 h后,擦掉小室上层细胞,用0.5%结晶紫溶液染色,显微镜下观察并拍照。 1.8 统计学处理
采用Graph Pad Prism 5.02统计软件,所有数据使用配对t检验进行分析,以x±s表示,检验水准(α)为0.05。
2 结 果 2.1 融合蛋白P-HNF4α的表达与纯化结果表明:构建的原核表达载体pET28a-P-HNF4α可表达带有His标签氨基端连接PEP-1的HNF4α融合蛋白(图 1A);发现24℃培养2 h所诱导的P-HNF4α表达量较17℃培养16 h高(图 1B);虽然0.5 mmol/L IPTG可诱导更多的P-HNF4α,但由于非特异条带表达量也较高(图 1B),给下游纯化带来难度,因此最佳的诱导条件确定为1.0 mmol/L IPTG、24℃培养2 h。将大量表达的含有P-HNF4α的细菌裂解液经亲和纯化、浓缩和透析后,获得了具有较高纯度的P-HNF4α,根据银染后灰度值计算,其纯度达到81%(图 1C)。
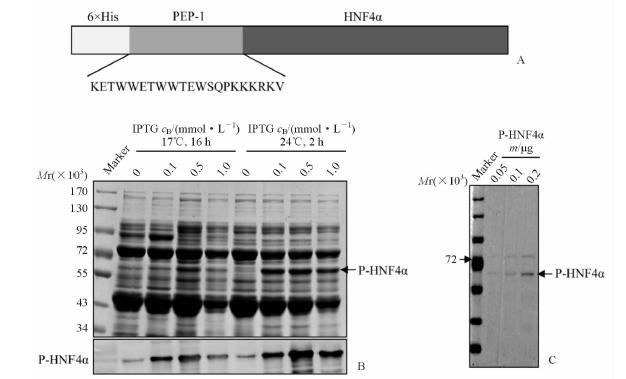
|
图 1 融合蛋白P-HNF4α的表达、纯化与鉴定 Fig 1 Expression,purification and identification of P-HNF4α A: Schematic illustration of fusion protein P-HNF4α (6×His-PEP-1-HNF4α). Amino acid sequence of PEP-1,KET WWE TWW TEW SQP KKR KV; B: Optimization of the prokaryotic expression conditions for P-HNF4α. Coomassie blue staining (top) and Western blotting analysis (bottom) showed the expression of P-HNF4α in designed conditions (0-1.0 mmol/L: concentration of IPTG); C: SDS-PAGE followed by silver staining was used to verify the purity of P-HNF4α protein,0.05,0.1,and 0.2 μg P-HNF4α protein were designed to run SDS-PAGE |
蛋白质印迹结果显示:随着浓度的升高,进入细胞的P-HNF4α也随之增加;当加入的P-HNF4α达到100 nmol/L时,外源HNF4α蛋白量超过了内源表达的HNF4α蛋白量(图 2A)。根据标准曲线计算100 nmol/L(5.9 μg)P-HNF4α转导Huh7细胞时的穿膜效率,5 μL细胞裂解液中所含P-HNF4α为0.318 1×(1.20+1.40)/2-0.002 1=0.41 (μg),因此,P-HNF4α进入细胞的效率为[0.41×(50/5)]/5.9×100%=69.5%(图 2B)。100 nmol/L终浓度的P-HNF4α转导Huh7细胞后,核质分离实验和细胞免疫荧光结果均显示P-HNF4α主要定位在细胞核内(图 2C、2D)。这些结果表明P-HNF4α可在穿膜肽的介导下进入肝癌细胞并定位于细胞核,提示其具备了在细胞核内发挥生物学功能的条件。

|
图 2 P-HNF4α转导Huh7细胞及其亚细胞定位 Fig 2 Transduction and sub-cellular localization of P-HNF4α in Huh7 cells A: P-HNF4α transduced into Huh7 cells. Different concentrations of P-HNF4α were transduced into Huh7 cells,and Western blotting analysis was processed to detect P-HNF4α (Input: 0.5 μg purified P-HNF4α; *: Degradation band of P-HNF4α; Endo HNF4α: Endogenous expression of HNF4α; CTR: Non-treated control; 10% glycerol: 10% glycerol/PBS control). B: Transduction efficiency of P-HNF4α. Western blotting analysis detected different amounts of purified P-HNF4α (0,0.1,0.2,0.3,0.4,and 0.5 μg) protein and the cell lysis from Huh7 cells transduced with P-HNF4α. The standard curve showed different amounts of P-HNF4α with gray value as the x axis and P-HNF4α (μg) as the y axis. C and D: Sub-cellular localization of P-HNF4α. Sub-cellular fractionation analysis (C) and immunocytochemistry assay (D) were done to determine the localization of P-HNF4α (100 nmol/L) transduced into Huh7 cells (Input: 0.2 μg purified P-HNF4α; WCL: Whole cell lysis; CE: Cytoplasmic extract; NE: Nuclear extract). Original magnification: ×400 (D) |
结果显示:与10%甘油处理的对照组相比,P-HNF4α转导可使受HNF4α调控的肝功能相关基因表达明显上调(P<0.05或0.01,图 3A),诱导恶性肝癌细胞向成熟的肝细胞分化;同时干细胞相关基因的表达下降(P<0.05或0.01,图 3B),表明肿瘤细胞的自我更新能力和高增殖活性可能受到抑制。这些结果提示P-HNF4α可能通过诱导分化肝癌细胞降低其恶性程度。
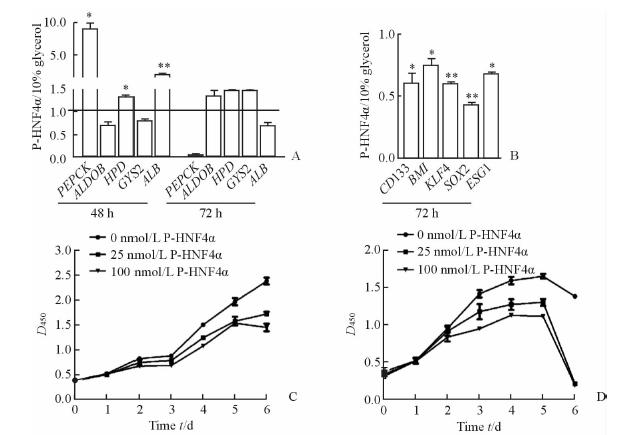
|
图 3 P-HNF4α对肝癌细胞基因表达和增殖的影响 Fig 3 Effect of P-HNF4α on mRNA expression and proliferation of hepatic cellular cancer (HCC) cells A and B: Gene expression of Huh7 cells transduced with P-HNF4α (100 nmol/L). The mRNA expression levels of characteristic hepatocyte markers (A) and “stemness” genes (B) in Huh7 cells were detected by real-time PCR. C and D: Effect of P-HNF4α on the proliferation of HCC cells. P-HNF4α inhibited the proliferation of Huh7 cells (C) and Hep3B cells (D). *P<0.05,**P<0.01; n=3,x±s |
进一步检测了P-HNF4α对肝癌细胞恶性表型的影响,包括肝癌细胞增殖能力、迁移能力以及侵袭能力。不同浓度P-HNF4α分别转导Huh7和Hep3B细胞,细胞生长曲线显示:与对照组(0 nmol/L)相比,25 nmol/L P-HNF4α转导肝癌细胞即可抑制其增殖,随着P-HNF4α浓度增加其细胞增殖的抑制效果更加显著(P<0.05,图 3C、3D);细胞划痕实验显示P-HNF4α可明显降低Huh7细胞的迁移(P<0.001,图 4A),小室侵袭实验表明P-HNF4α抑制了Huh7的侵袭能力(P<0.05,图 4B)。
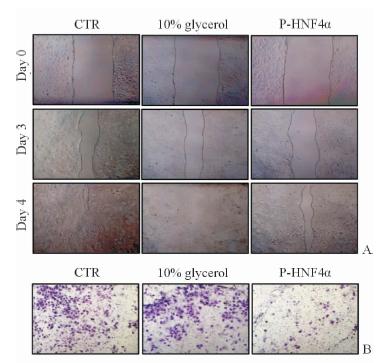
|
图 4 P-HNF4α对肝癌细胞迁移和侵袭的影响 Fig 4 Effect of P-HNF4α on migration and invasion of HCC cells The wound-healing assay (A) and trans well invasion assay (B) showed the fusion protein P-HNF4α (100 nmol/L) significantly attenuated the migration and invasion ability of Huh7 cells,indicating the inhibition effect of P-HNF4α on tumor malignancy. Original magnification: ×40(A),×100(B) |
HNF4α是肝脏胚胎期肝上皮细胞形成和肝脏成熟期肝细胞上皮性状保持所必需的核因子[4, 5],与肝脏结构的构成、肝脏上皮细胞的产生,以及肝细胞的形态和功能分化密切相关[4, 5, 6]。HNF4α可在转录水平调控下游多种基因的表达,有效维持和改善肝细胞分化水平,增强肝细胞蛋白合成、糖原合成、药物代谢以及解毒功能。在肝癌的发生发展过程中HNF4α表达下降,其表达下调是肝癌发生的始动因素。我们前期研究显示,腺病毒介导的过表达HNF4α可降低癌细胞的成瘤性,小鼠尾静脉注射携带HNF4α基因的腺病毒可有效抑制肝脏转移瘤的形成,瘤内注射HNF4α腺病毒也表现出显著的抗肿瘤效果[1]。因此,调控HNF4α表达可能成为治疗肝癌的有效途径。
腺病毒作为基因治疗的研究热点,其临床应用存在争议。病毒一旦进入细胞便成为人为无法控制的因素,可能在宿主细胞内对宿主基因进行无法预计的修饰;同时病毒所携带的基因转录翻译为功能蛋白的效率不可控。而以多肽和脂类为基础的载体因其具有操作简单、毒性小、免疫原性较低以及无插入突变等优点,被越来越广泛地研究[7]。众多研究表明,CPPs是一类可高效穿膜的非病毒型载体。穿膜肽多聚精氨酸(poly-arginine,11R)可成功介导抑癌蛋白p53进入膀胱癌细胞和神经胶质瘤细胞,并抑制了细胞增殖[8, 9]; 带11R的融合蛋白Oct4-11R、Klf4-11R、Sox2-11R、c-MYC-11R同时转入小鼠胚胎成纤维细胞后诱导产生了诱导多能干细胞[10]。这些研究表明穿膜肽可介导核蛋白进入细胞发挥功能。穿膜肽的应用不仅限于细胞学实验,其在体内的应用也在不断尝试中显现出了重要价值。用带穿膜肽Tat的p53和Smac融合蛋白处理荷瘤小鼠模型,都发现可显著延长小鼠的存活时间[11, 12]。PEP-1相比于其他穿膜肽有着独有的优势,PEP-1能携带完整活性的多肽和蛋白进入细胞;而且其在作为载体介导蛋白药物治疗方面也具有明显优点,如在生理缓冲液中稳定性高、毒性低、对血清不敏感等[13],并能在极短的时间内携带蛋白药物进入细胞。PEP-1介导蛋白药物在神经胶质瘤和肠道缺血疾病治疗中已显示了极大潜能[14, 15]。
为了拓展HNF4α诱导分化治疗肝癌的新方法,本研究利用穿膜肽PEP-1为载体,携带HNF4α蛋白进入肝癌细胞,并确定了其对肝癌细胞的作用。研究结果表明PEP-1介导的HNF4α蛋白转导肝癌细胞,穿膜效率达到69.5%,P-HNF4α转导进入细胞后主要定位在肝癌细胞的细胞核中(图 2C、图 2D),并可促进肝脏功能基因的表达,抑制干性相关基因的表达(图 3A、图 3B),显示该重组蛋白具有HNF4α功能,可在细胞内发挥肝细胞核因子的作用,通过促进肝癌细胞向成熟肝细胞分化从而诱导分化肝癌细胞,降低了肝癌细胞的恶性程度;而且P-HNF4α能有效抑制肝癌细胞增殖,在25 nmol/L P-HNF4α作用下细胞增殖明显受到抑制(图 3C、图 3D),说明低浓度的P-HNF4α即可对肝癌细胞发挥作用;细胞划痕实验和小室侵袭实验也表明P-HNF4α有效抑制了肝癌细胞Huh7的迁移和侵袭性(图 4),提示P-HNF4α可降低肝癌细胞的转移能力。这些结果显示P-HNF4α转导具有治疗肝癌的极大潜能。但P-HNF4α对肝癌发展的逆转作用,还需体内实验进一步证实。
| [1] | Yin C, Lin Y, Zhang X, Chen Y X, Zeng X, Yue H Y, et al. Differentiation therapy of hepatocellular carcinoma in mice with recombinant adenovirus carrying hepatocyte nuclear factor-4α gene[J].Hepatology, 2008, 48:1528-1539. |
| [2] | Patel L N, Zaro J L, Shen W C. Cell penetrating peptides: intracellular pathways and pharmaceutical perspectives[J].Pharm Res,2007,24:1977-1992. |
| [3] | Chandra V, Hang P, Potluri N, Wu D, Kim Y, Rastinejad F. Multidomain integration in the structure of the HNF4α nuclear receptor complex[J].Nature,2013,495:394-398. |
| [4] | Parviz F, Matullo C, Garrison W D, Savatski L, Adamson J W, Ning G. Hepatocyte nuclear factor 4α controls the development of a hepatic epithelium and liver morphogenesis[J].Nat Genet,2003,34:292-296. |
| [5] | Battle M A, Konopka G, Parviz F, Gaggl A L, Yang C, Sladek F M. Hepatocyte nuclear factor 4α orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver[J].Proc Natl Acad Sci USA,2006,103:8419-8424. |
| [6] | Watt A J, Garrison W D, Duncan S A. HNF4: a central regulator of hepatocyte differentiation and function[J].Hepatology,2003, 37:1249-1253. |
| [7] | Huang Y W, Lee H J, Tolliver L M, Aronstam R S. Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: opportunities and challenges[J].Biomed Res Int,2015,2015:834079. |
| [8] | Inoue M, Tomizawa K, Matsushita M, Lu Y F, Yokoyama T, Yanai H, et al. p53 protein transduction therapy: successful targeting and inhibition of the growth of the bladder cancer cells[J].Eur Urol,2006,49:161-168. |
| [9] | Michiue H, Tomizawa K, Wei F Y, Matsushita M, Lu Y F, Ichikawa T, et al. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction[J]. J Biol Chem,2005,280:8285-8289. |
| [10] | Zhou H, Wu S, Joo J Y, Zhu S, Han D W, Lin T, et al. Generation of induced pluripotent stem cells using recombinant proteins[J]. Cell Stem Cell, 2009:381-384. |
| [11] | Snyder E L, Meade B R, Saenz C C, Dowdy S F. Treatment of terminal peritoneal carcinomatosis by a transducible p53-activating peptide[J]. PLoS Biol,2004,2:E36. |
| [12] | Fulda S, Wick W, Weller M, Debatin K M. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo[J]. Nat Med,2002,8:808-815. |
| [13] | Morris M C, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells[J].Nat Biotechnol,2001,19:1173-1176. |
| [14] | Wang B, Lv L, Wang Z, Zhao Y, Wu L, Fang X, et al. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2-mediated endocytosis[J].Biomaterials, 2014, 35:5897-5907. |
| [15] | He X H, Yan X T, Wang Y L, Wang C Y, Zhang Z Z, Zhan J. Transduced PEP-1-heme oxygenase-1 fusion protein protects against intestinal ischemia/reperfusion injury[J]. J Surg Res,2014, 187:77-84. |
 2015, Vol. 36
2015, Vol. 36


