2. 第二军医大学长海医院泌尿外科, 上海 200433;
3. 第二军医大学长海医院病理科, 上海 200433
2. Department of Urology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China;
3. Department of Pathology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
肾细胞癌(renal cell carcinoma,RCC)是泌尿系统常见的恶性肿瘤,随着人们健康意识的增强和体检的普及,近年来肾癌整体发病率呈逐年上升趋势,平均每年增幅为2%~3%[1]。目前有相当部分的肾癌是由体检发现的早期肾癌,其治疗方式以手术为主。早期肾癌预后好,T1期肾癌的5年生存率可达90%以上[2, 3]。但是仍有相当部分的早期肾癌术后会出现复发转移,转移患者5年生存率很低[4, 5]。寻找可以预测早期肾癌预后的指标,为手术后的个性化治疗提供方案非常必要。
真核翻译延伸因子1A1(eukaryotic translation elongation factor 1A1,eEF1A1)是参与蛋白质翻译过程中的一个重要蛋白,在细胞生长、组织发育过程中起着重要作用,是细胞生长过程中不可或缺的蛋白[6]。近年来大量研究表明eEF1A1除了参与翻译过程之外,还和细胞骨架蛋白调节、细胞增殖和凋亡以及迁移有着重要关系,具有潜在的致癌特性[7, 8, 9, 10, 11]。探讨eEF1A1和肿瘤有关的功能和机制,有利于研究的深入开展。
本研究通过免疫组化技术检测T1N0M0期肾癌组织中eEF1A1的表达,分析eEF1A1表达与Fuhrman病理分级及预后之间的关系,初步探讨eEF1A1能否作为早期肾癌预后指标。 1 材料和方法 1.1 标本来源
收集第二军医大学长海医院泌尿外科2007年全年肾癌手术患者资料及标本。入选标准:首次发现肾癌且行肾癌根治术治疗;术前未行其他治疗;术后证实TNM分期为T1N0M0(肿瘤≤7 cm,无淋巴结转移,无远处转移);能获得病理组织;术后随访资料完整。所有符合要求的患者一共70例。患者中男性45例,女性25例;<60岁24例,≥60岁46例,平均年龄(55.74±11.49)岁 ;左侧32例,右侧38例; Fuhrman分级Ⅰ、Ⅱ、Ⅲ、Ⅳ级分别为14、40、15和1例。肿瘤平均大小(4.8±1.4) cm,患者在术后1个月、3个月、1年进行复查随访。以后根据患者肿瘤情况不同,每半年或1年复查随访。标本采集均经过患者及家属同意并签署知情同意书,经长海医院伦理委员会审核通过。 1.2 方法
对每例患者的标本进行eEF1A1免疫组化染色。标本经10%甲醛固定,常规石蜡包埋,用石蜡切片机修片、切片,常规脱蜡、水化,Tris-EDTA (pH 8.0) 沸水浴40 min进行抗原修复后自然冷却至室温,用磷酸盐缓冲液(PBS)冲洗,3%牛血清白蛋白(BSA)室温封闭60 min,加入eEF1A1 小鼠抗人抗体(sc-21758,Santa Cruz),室温孵育过夜。PBS冲洗,滴加辣根过氧化物酶标记的二抗,室温75 min,PBS冲洗,DAB显色,苏木精复染细胞核,乙醇脱水,二甲苯透明,中性树胶封片。病理科医生进行读片判断eEF1A1的表达量,eEF1A1阳性为棕黄色颗粒,核为蓝紫色颗粒。找出片中eEF1A1表达量较高的区域,棕褐色和深黄色记为阳性(+),浅黄色或不着色记为阴性(-)。 1.3 统计学处理
采用SPSS 22.0进行统计学分析。eEF1A1高表达和低表达与年龄、肿瘤侧别、肿瘤Fuhrman分级、术前是否有局部症状之间的关系采用χ2检验进行比较,对期望值小于5的采用Fisher精确概率法进行比较。生存分析采用Kaplan-Meier法并进行log-rank检验。检验水准(α)为0.05。 2 结 果 2.1 随访结果
入组70例患者定期随访,随访资料完整。随访时间为4~95个月,平均(77.9±18.3)个月,57例患者未出现复发转移,62例患者(88.6%)存活。 2.2 免疫组化结果与临床资料的关系
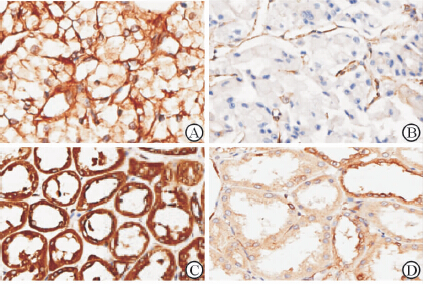
免疫组化结果如图 1所示,低表达细胞eEF1A1主要定位于胞质中,并且着色较淡;高表达细胞eEF1A1同时定位于胞核和胞质中,着色为深褐色。70例癌组织eEF1A1高表达43例,低表达27例,高表达率为61.4%;,癌旁组织eEF1A1高表达39例,低表达31例,高表达率55.7%。结合患者临床资料进行交叉表χ2检验,如表 1所示,癌组织中eEF1A1表达量高低与患者性别、年龄、肿瘤的左右侧均无关,而与肿瘤大小及Fuhrman分级有关(P<0.05)。>4 cm、Fuhrman分级为Ⅲ~Ⅳ级的患者癌组织中,eEF1A1高表达者所占比例较高(P<0.05)。术前出现局部症状的患者癌组织中eEF1A1表达量较高(P=0.042)。同时,癌组织中eEF1A1表达量的高低还与预后有关(P=0.020):癌组织低表达eEF1A1患者尚未出现死亡病例,而癌织组高表达eEF1A1的则有8例患者死亡。
|
图 1 免疫组织化学法检测肾癌组织中eEF1A1的表达 Fig 1 eEF1A1 expression in renal tissues of patients with renal cell carcinoma detected by immunohistochemistry A: eEF1A1 high expression in cancer tissue; B: eEF1A1 low expression in cancer tissue; C: eEF1A1 high expression in adjacent tissue; D: eEF1A1 low expression in adjacent tissue. Original magnification: ×200 |
|
|
表 1 癌组织eEF1A1表达高低与临床特征的关系 Tab 1 Relationship between the expression of eEF1A1 and patient clinical data |
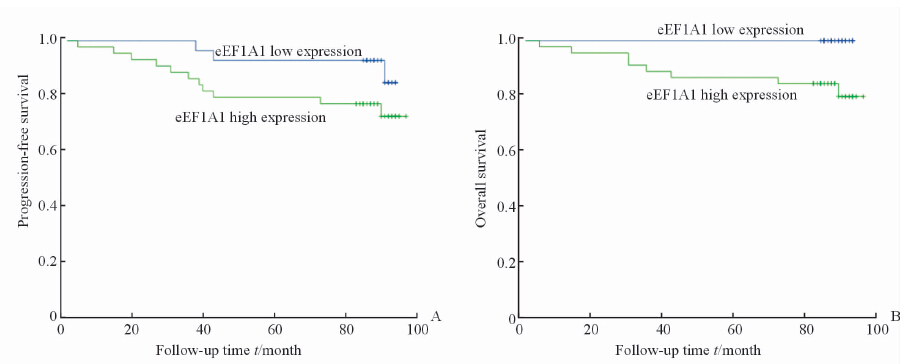
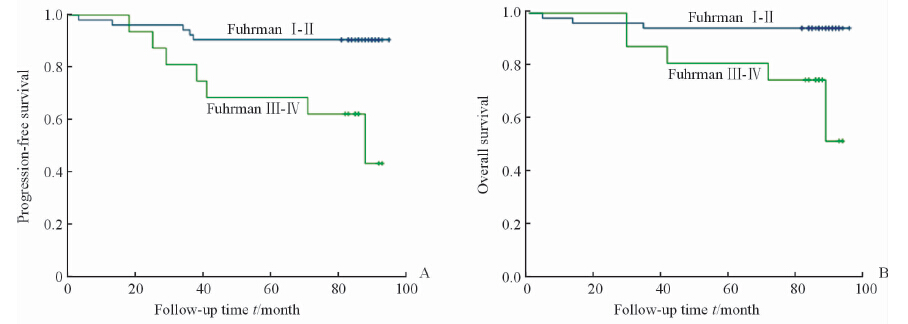
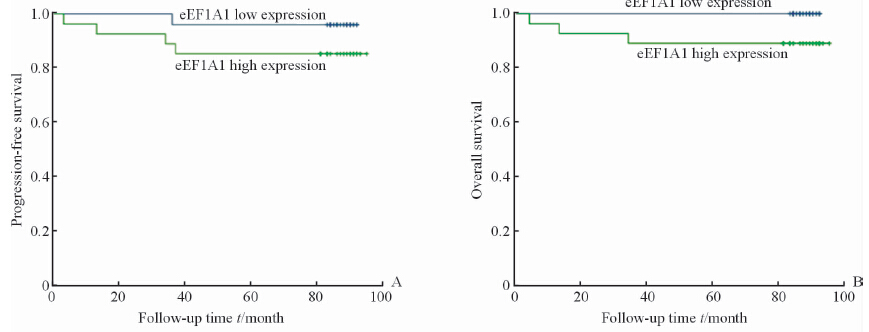
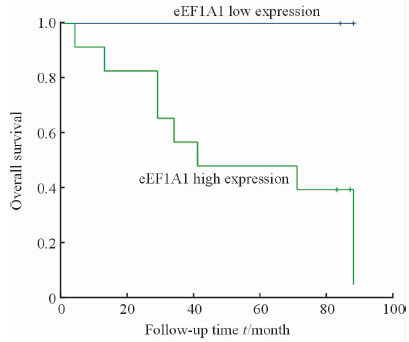
将样本分为两组:eEF1A1高表达和eEF1A1低表达。根据两组的复发转移和生存情况进行Kaplan-Meier分析并绘制生存曲线,进行log-rank检验,获得患者无进展生存情况及总体生存率。结果如图 2示,显示癌组织中eEF1A1高表达组出现复发、转移的患者较多,但是可能由于样本量较小,差异无统计学意义(P=0.118)。生存分析显示eEF1A1低表达组患者生存时间更长,且差异有统计学意义(P=0.019),eEF1A1低表达患者更是在随访期内未出现死亡。Fuhrman分级Ⅰ~Ⅱ级患者的无进展生存和总体生存情况均较Fuhrman分级Ⅲ~Ⅳ级患者更好(P=0.001,P=0.004),如图 3所示。本研究中,Fuhrman Ⅲ~Ⅳ的患者只有1例癌组织eEF1A1低表达,且未发生复发转移。而Fuhrman分级Ⅰ~Ⅱ级的患者中依然出现了eEF1A1低表达患者生存情况略好的趋势(P=0.09),如图 4所示。有意思的是,在出现了复发转移的患者中,eEF1A1低表达的患者在随访期间尚未出现死亡,治疗情况较好(P=0.095),如图 5所示。由于纳入研究的患者死亡率并不高,这两项统计分析中P值略大于0.05,需要进一步的验证。
|
图 2 患者无进展生存期(A)和总生存期(B)与eEF1A1表达量间的关系 Fig 2 Relationship of eEF1A1 expression with progression-free survival (A) and overall survival period (B) |
|
图 3 患者无进展生存期(A)和总生存期(B)与Fuhrman分级间的关系 Fig 3 Relationship of Fuhrman grade with progression-free survival (A) and overall survival period (B) |
|
图 4 Fuhrman Ⅰ~Ⅱ级患者无进展生存期(A)和总生存期(B) Fig 4 Progression-free survival period (A) and overall survival period (B) of Fuhrman Ⅰ-Ⅱ patients |
|
图 5 复发、转移患者的生存情况 Fig 5 Overall survival of patients with metastasis or recurrence |
近年来,随着经济的发展和人们健康意识的进步,体检越来越普及,很多肾肿瘤在体检中被发现。早期肾癌整体预后较好,有研究指出早期肾癌术后5年生存率超过90%[2, 3],而本研究经过平均77.9(4~95)个月的随访,62例患者(88.6%)存活,其中57例患者(80.0%)无复发转移。然而,仍有13例患者(20%)出现了复发转移,肾癌一旦复发尚无有效的治疗方法。肾细胞癌主要的病理类型为肾透明细胞癌,对放疗和化疗不敏感,以IL-2和IFN-α为主的细胞因子治疗以及以索拉非尼、舒尼替尼为代表的靶向治疗效果也并不理想。因此,如果能够在术后立即判断肾癌的预后并且提前采取相应措施,在精确医疗的发展中显得十分重要。寻找能够预示早期肾癌预后的指标是重要方法之一。目前比较经典的肾癌预后指标是Fuhrman分级,Fuhrman Ⅲ级和Ⅳ级的肾癌预后与Ⅰ级和Ⅱ级相比明显较差。近年来有也有一些预后因子,如胰岛素样生长因子Ⅱ mRNA结合蛋白3(insulin-like growth factorⅡmRNA binding protein 3,IMP3)[12, 13]、免疫共刺激分子(B7 homolog 1,B7-H1)[14, 15]等。
本研究初步探讨eEF1A1和早期肾癌预后的关系。蛋白质的产生可分为转录、翻译两大步骤,而翻译又可以分为起始、延长和中止3个阶段,eEF1A在蛋白质翻译过程中起着非常重要的作用。eEF1A有两个不同的亚型分别为eEF1A1和eEF1A2,分别由6号染色体和20号染色体上的两个基因编码。eEF1A1和eEF1A2约有97%的编码是同源的。一般认为生理状态下,这两种异构体有组织特异性表达,这两种亚型不能在同一组织内表达[16],eEF1A2仅在神经元和肌肉细胞内表达,而eEF1A1则在人体各个器官内表达[17, 18]。近年来发现eEF1A1不仅在蛋白质翻译过程中起重要作用,而且通过其他途径影响细胞增殖、凋亡和迁移、侵袭,与肿瘤的发生发展密切相关[16, 19, 20]。当细胞内液pH呈碱性时,细胞增殖能力增强,而运用RNA干扰技术将eEF1A1沉默后这一现象消失,进一步研究发现,这种现象可能和eEF1A1在碱性环境中与细胞骨架蛋白的结合力减弱有关[21]。eEF1A1还发挥着膜受体的功能,当停留在细胞膜上的eEF1A1增多时,其可以识别纤维连接蛋白上的“失巢凋亡”相关位点而诱导凋亡。而在肿瘤细胞中,这种调节机制被打破,使细胞凋亡减少[22]。上皮间质化(epithelial mesenchymal transition,EMT)是肿瘤迁移和转移过程中的一个重要过程,有研究发现eEF1A1与核内不均一核糖蛋白E1(heterogeneous nuclear ribonucleoprotein E1,hnRNP E1)结合可以抑制EMT相关基因翻译的沉默从而抑制EMT[23]。另外,eEF1A1可以和p53家族蛋白结合,从而抑制p53介导的促凋亡作用[10]。顺铂、甲氨蝶呤等化疗药的耐药性可能与高表达eEF1A1相关,结肠癌、乳腺癌、胰腺癌的耐药细胞株中均发现了eEF1A1的高表达[24]。研究发现eEF1A1在白血病、肝癌、前列腺癌和乳腺癌等恶性肿瘤中呈高表达[25, 26, 27],但eEF1A1表达与肾癌的关系尚无相关报道。
本研究首次报道eEF1A1在肾癌中表达量与预后的关系,发现肾癌组织eEF1A1表达量的高低和肾癌的Fuhrman分级有关,Fuhrman分级高的肾癌中eEF1A1相对高表达。生存分析则显示肾癌组织中eEF1A1高表达直接影响到患者的生存期(P<0.05)。癌组织中eEF1A1表达量低的患者均存活,而8名死亡患者eEF1A1表达量均高。在Fuhrman分级Ⅰ~Ⅱ级的患者中,癌组织低表达患者生存期更长,提示eEF1A1有望和Fuhrman分级等预后指标合并更精确地预测预后。在14例出现复发转移的患者中,癌组织中eEF1A1低表达的患者生存期更长,提示在晚期肾癌患者中,eEF1A1或许可用于预测治疗效果。因此,eEF1A1表达量低有望作为一个预后良好的标志物。本研究中,eEF1A1在43例患者的癌组织中高表达,死亡率并不高。从生存分析也可看出,即使对于eEF1A1高表达的早期肾癌患者,在经过了平均超过6年的随访后,中位生存期和无进展中位生存期均未达到。因此,eEF1A1高表达并不一定预示着预后差,而低表达可能预示着预后良好。从eEF1A1的功能考虑,肿瘤细胞的增殖、迁移和侵袭可能需要克服更多的障碍,而eEF1A1参与蛋白质合成、细胞骨架蛋白功能调节,可能在起始过程中发挥着重要作用。eEF1A1可能作为肾癌细胞复发转移一个必要条件,是否可以作为治疗的一个靶点,需要进一步进行机制研究。
总之,eEF1A1作为体内一种重要的管家基因,其表达变化可能和肾癌的预后有关,结合Fuhrman分级,其可能作为预测早期肾癌预后的一个指标以及用于转移性肾癌治疗效果的研究。但本研究的样本量有限,尤其是出现终点事件(复发、转移或死亡)的患者较少,还需要更大样本的前瞻性研究来证实这一结论,并进一步进行基础实验探索eEF1A1对转移性肾癌的影响及机制。
| [1] | Siegel R L, Miller K D,Jemal A. Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65:5-29. |
| [2] | Springer C, Hoda M R, Fajkovic H, Pini G, Mohammed N, Fornara P, et al. Laparoscopic vs open partial nephrectomy for T1 renal tumours: evaluation of long-term oncological and functional outcomes in 340 patients[J]. BJU Int, 2013, 111:281-288. |
| [3] | Ha Y S, Park Y H, Kang S H, Hong S H, Hwang T K, Byun S S, et al. Predictive factors for late recurrence in patients with stage T1 clear cell renal cell carcinoma: a multiinstitutional study[J]. Clin Genitourin Cancer, 2013, 11:51-55. |
| [4] | Lam J S, Shvarts O, Leppert J T, Pantuck A J, Figlin R A, Belldegrun A S. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system[J]. J Urol, 2005, 174:466-472. |
| [5] | Zisman A, Pantuck A J, Dorey F, Said J W, Shvarts O, Quintana D, et al. Improved prognostication of renal cell carcinoma using an integrated staging system[J]. J Clin Oncol, 2001, 19:1649-1657. |
| [6] | Kapp L D,Lorsch J R. The molecular mechanics of eukaryotic translation[J]. Annu Rev Biochem, 2004, 73:657-704. |
| [7] | Doyle A, Crosby S R, Burton D R, Lilley F,Murphy M F. Actin bundling and polymerisation properties of eukaryotic elongation factor 1 alpha (eEF1A), histone H2A-H2B and lysozyme in vitro[J].J Struct Biol, 2011, 176:370-378. |
| [8] | Pittman Y R, Kandl K, Lewis M, Valente L,Kinzy T G. Coordination of eukaryotic translation elongation factor 1A (eEF1A) function in actin organization and translation elongation by the guanine nucleotide exchange factor eEF1Balpha[J]. Biol Chem,2009,284:4739-4747. |
| [9] | Pecorari L, Marin O, Silvestri C, Candini O, Rossi E, Guerzoni C, et al. Elongation factor 1 alpha interacts with phospho-Akt in breast cancer cells and regulates their proliferation, survival and motility[J]. Mol Cancer, 2009, 8:58. |
| [10] | Blanch A, Robinson F, Watson I R, Cheng L S,Irwin M S. Eukaryotic translation elongation factor 1-alpha 1 inhibits p53 and p73 dependent apoptosis and chemotherapy sensitivity[J]. PLoS One, 2013, 8:e66436. |
| [11] | Gross S R,Kinzy T G. Translation elongation factor 1A is essential for regulation of the actin cytoskeleton and cell morphology[J]. Nat Struct Mol Biol, 2005, 12:772-778. |
| [12] | Jiang Z, Chu P G, Woda B A, Liu Q, Balaji K C, Rock K L, et al. Combination of quantitative IMP3 and tumor stage: a new system to predict metastasis for patients with localized renal cell carcinomas[J]. Clin Cancer Res, 2008, 14:5579-5584. |
| [13] | Hoffmann N E, Sheinin Y, Lohse C M, Parker A S, Leibovich B C, Jiang Z, et al. External validation of IMP3 expression as an independent prognostic marker for metastatic progression and death for patients with clear cell renal cell carcinoma[J]. Cancer, 2008, 112:1471-1479. |
| [14] | Patel S P,Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy[J]. Mol Cancer Ther, 2015, 14:847-856. |
| [15] | Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Martignoni G, et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises[J]. Cancer Treat Rev, 2015, 41:114-121. |
| [16] | Soares D C, Barlow P N, Newbery H J, Porteous D J,Abbott C M. Structural models of human eEF1A1 and eEF1A2 reveal two distinct surface clusters of sequence variation and potential differences in phosphorylation[J]. PLoS One, 2009, 4:e6315. |
| [17] | Newbery H J, Loh D H, O’donoghue J E, Tomlinson V A, Chau Y Y, Boyd J A, et al. Translation elongation factor eEF1A2 is essential for post-weaning survival in mice[J]. J Biol Chem, 2007, 282:28951-28959. |
| [18] | Abbott C M, Newbery H J, Squires C E, Brownstein D, Griffiths L A, Soares D C. eEF1A2 and neuronal degeneration[J]. Biochem Soc Trans, 2009, 37:1293-1297. |
| [19] | Andersen G R, Pedersen L, Valente L, Chatterjee I, Kinzy T G, Kjeldgaard M, et al. Structural basis for nucleotide exchange and competition with tRNA in the yeast elongation factor complex eEF1A:eEF1B alpha[J]. Mol Cell, 2000, 6:1261-1266. |
| [20] | Moore R C, Durso N A,Cyr R J. Elongation factor-1alpha stabilizes microtubules in a calcium/calmodulin-dependent manner[J]. Cell Motil Cytoskeleton, 1998, 41:168-180. |
| [21] | Kim J, Namkung W, Yoon J S, Jo M J, Lee S H, Kim K H, et al. The role of translation elongation factor eEF1A in intracellular alkalinization-induced tumor cell growth[J]. Lab Invest, 2009, 89:867-874. |
| [22] | Itagaki K, Naito T, Iwakiri R, Haga M, Miura S, Saito Y, et al. Eukaryotic translation elongation factor 1A induces anoikis by triggering cell detachment[J]. J Biol Chem, 2012, 287:16037-16046. |
| [23] | Hussey G S, Chaudhury A, Dawson A E, Lindner D J, Knudsen C R, Wilce M C, et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step[J]. Mol Cell, 2011, 41:419-431. |
| [24] | Selga E, Oleaga C, Ramirez S, De Almagro M C, Noe V, Ciudad C J. Networking of differentially expressed genes in human cancer cells resistant to methotrexate[J]. Genome Med, 2009, 1:83. |
| [25] | Grassi G, Scaggiante B, Farra R, Dapas B, Agostini F, Baiz D, et al. The expression levels of the translational factors eEF1A 1/2 correlate with cell growth but not apoptosis in hepatocellular carcinoma cell lines with different differentiation grade[J].Biochimie,2007, 89:1544-1552. |
| [26] | Edmonds B T, Wyckoff J, Yeung Y G, Wang Y, Stanley E R, Jones J, et al. Elongation factor-1 alpha is an overexpressed actin binding protein in metastatic rat mammary adenocarcinoma[J]. J Cell Sci, 1996, 109:2705-2714. |
| [27] | Rehman I, Evans C A, Glen A, Cross S S, Eaton C L, Down J, et al. iTRAQ identification of candidate serum biomarkers associated with metastatic progression of human prostate cancer[J]. PLoS One, 2012, 7:e30885. |
 2015, Vol. 36
2015, Vol. 36


