糖尿病肾病是长期糖尿病的重要并发症之一,是引起终末期肾脏疾病的主要原因[1],已得到公认。但肾脏疾病诱发糖尿病的相关证据目前较少。我们的前期研究发现左肾切除大鼠术后6个月会出现慢性肾脏疾病[2, 3],随之出现糖代谢异常[4]。结果表明单肾切除所致肾功能损伤导致了大鼠糖代谢异常,提示肾功能损伤可能也是糖尿病的重要病因,但目前机制尚不清楚。
肾素-血管紧张素系统(renin-antiotensin system,RAS)持续激活是引起肾脏疾病进展的重要原因[5]。前期研究发现单肾切除大鼠体内存在RAS的持续激活,且血管紧张素转化酶抑制剂(angiotensin converting enzyme inhibitor,ACEI)能在一定程度上改善单肾切除所致的糖代谢异常[4],提示肾脏RAS持续激活可能与肾脏疾病引起的大鼠糖代谢异常有关。腺苷酸活化蛋白激酶(AMP-activated protein kinase,AMPK)是能量代谢的总开关,且在肾脏组织中高表达[6],与RAS系统具有潜在的联系。因此,本研究在前期研究的基础上,进一步采用ACEI及血管紧张素受体阻滞剂(angiotensin receptor blocker,ARB)来干预单侧肾切除大鼠,观察大鼠肾功能、糖代谢相关指标及AMPK表达的变化,探讨肾功能损害导致糖代谢异常可能的作用机制。 1 材料和方法 1.1 动物来源及分组
Sprague-Dawley雄性大鼠40只,体质量300~350 g,由桂林医学院实验动物中心提供,每2只大鼠关在同一笼里,室温(23±1)℃,每天12 h光照,给予标准实验室饮食,不控制饮水量。大鼠随机均分为4组:单肾切除组(uinephrectomy,UNX)、假手术组(Sham)、ACEI治疗组、ARB治疗组(n=10)。所有大鼠采用氯胺酮(75 mg/kg; Alfasan,Woerden,Holland)麻醉。UNX组大鼠行左侧腹部1~1.5 cm切口,取出左肾,保持肾上腺完整;Sham组大鼠行左侧腹部1~1.5 cm切口但不取左肾;ACEI治疗组和ARB治疗组大鼠先行左肾切除术,然后将赖诺普利(A0773,Sigma-Aldrich,Inc.,USA)或洛沙坦(Y0001076,Sigma-Aldrich,Inc.,USA)溶解于无菌蒸馏水中,每天按4 mg/kg剂量灌胃。UNX组和Sham组大鼠,每天摄入3 mL蒸馏水作为安慰剂对照。本实验历经10个月,获桂林医学院动物实验伦理委员会批准。 1.2 生化和代谢指标检测 1.2.1 糖代谢相关指标
术后3、6、8、10个月时,大鼠禁食8 h后,取尾静脉血用快速血糖仪(OneTouch Ultra,LifeScan,Inc. USA)检测血糖作为空腹血糖(mmol/L);采集心脏空腹血样、离心,用ELISA试剂盒(Enzyme immunoassay,Mercodia,Sweden)检测胰岛素浓度(mU/L),具体步骤:酶偶联抗体和显色底物TMB先后加入受检血清中,经过孵育和洗涤,在光密度450 nm处用ELISA分析仪(μQuant,Bio-Tek Instruments Inc.,America)检测胰岛素浓度(mU/L)。胰岛素抵抗指数(homeostasis of model assessment-insulin resistance,HOMA-IR)=空腹血糖(mmol/L)×空腹胰岛素(mU/L)/22.5。 1.2.2 肾功能相关指标
术后10个月时,用代谢笼收集大鼠24 h尿量,用于检测尿总蛋白/肌酐比值。然后采集大鼠心脏空腹血样,检测血尿素氮和血肌酐等反映肾功能的指标。血尿素氮(酶法),血清/尿肌酐(Jaffe动力学法)和尿总蛋白(免疫比浊法)均在同一生化分析仪上检测(C501,Roche Diagnostics GmbH,Mannheim,Germany)。 1.3 蛋白免疫印迹检测AMPK蛋白表达
术后10个月时,各组大鼠均被处死,取出右侧肾脏。将肾皮质溶于缓冲液50 mmol/L Tris-HCl (pH 7.4),150 mmol/L NaCl,1 mmol/L苯甲基磺酰氟,1 mmol/L EDTA,1%脱氧胆酸钠,1%Triton X-100,1%十二烷基硫酸钠和5%蛋白酶抑制剂(cat no. P2714; Sigma,St Louis,MO),匀浆4℃下13 000 r/min(r=8 cm)离心10 min,分离上清液,上清液中蛋白浓度用定量试剂盒测定(cat no. 23225; ThermoFisher Scientific,Waltham,MA)。将100 mg组织溶解产物和预染的相对分子质量标记物(Bio-Rad,Hercules,CA)于PAGE凝胶上(4%丙烯酰胺叠加凝胶和8%分离凝胶)进行电泳,将分离的蛋白条带转移至硝酸纤维素膜上。硝酸纤维素膜先在室温下用5%脱脂牛奶封闭1 h,然后加入含山羊抗phospho-AMPK α1抗体(Ser 496)∶sc-101631(1∶200); Santa Cruz Biotechnology的TBS(0.05% Tween 20,TBS-T)和5%脱脂牛奶 于4℃孵育12 h,洗涤,最后加偶联辣根过氧化物酶(1∶2 000; Upstate,Temecula,MA)的二抗,用电化学发光法(Amersham,Piscataway,NJ)检测显色蛋白条带的免疫反应强度。为准确判断待测蛋白的表达情况,向电泳槽上同时加入内参。内参β-actin和AMPK的相对分子质量大小分别约为43 000、74 000。 1.4 免疫荧光法测定肾脏组织AMPK表达
术后10个月时,大鼠右侧肾脏被取出,用于免疫荧光染色,观察各组大鼠肾皮质AMPK表达的差异。肾组织于液氮中固定24 h后用石蜡包埋并切成4 μm的薄片,接着用1%牛血清白蛋白封闭30 min,然后加山羊抗phospho-AMPK α1,4℃孵育12 h后洗涤,最后加偶联Alexa 488(绿色)的抗山羊二抗(1∶200),室温孵育1 h后洗涤,于荧光显微镜下观察(AX10,Carl Zeiss,Hamburg,Germany)。 1.5 统计学处理
采用SPSS 13.0软件,方差分析各组大鼠空腹血糖和血胰岛素,检验水准(α)为0.05。 2 结 果 2.1 RAS阻滞剂对单肾切除大鼠肾功能的影响
结果(图 1)表明:术后10个月时,UNX大鼠血尿素氮、血肌酐及尿总蛋白/肌酐比值均较Sham组大鼠升高[(17.9±3.6) vs (6.8±1.7) mmol/L,(88.5±16.3) vs (40.6±10.6) μmol/L,(0.651±0.158) vs (0.385±0.141) mg/mmol],组间差异有统计学意义(P<0.05);ACEI治疗组及ARB治疗组血尿素氮[(7.0±1.6)、(8.7±1.8) mmol/L]、血肌酐[(43.4±13.1)、(45.8±10.4) μmol/L]及尿总蛋白/肌酐比值均较UNX组[(0.150±0.119)、(0.276±0.159) mg/mmol]降低,组间差异有统计学意义(P<0.05)。
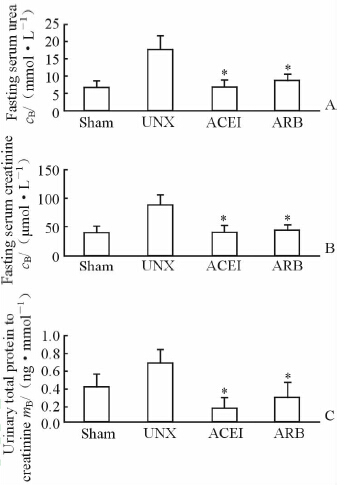
|
图 1 RAS阻滞剂对单肾切除大鼠肾功能的影响 Fig 1 Influence of RAS blockade on renal dysfunction in uninephrectomized rats A: Fasting sreum urea; B: Fasting serum creatinine; C: Urinary total protein to creatinine. *P<0.05 vs UNX group; n=10,x±s |
结果(图 2A)表明:与其他3组相比,UNX组大鼠空腹血糖明显升高。术后8个月,UNX组空腹血糖高于Sham组[(5.9±0.3) vs (5.3±0.6) mmol/L],差异有统计学意义(P<0.05);ACEI治疗组和ARB治疗组空腹血糖与Sham组差异无统计学意义。
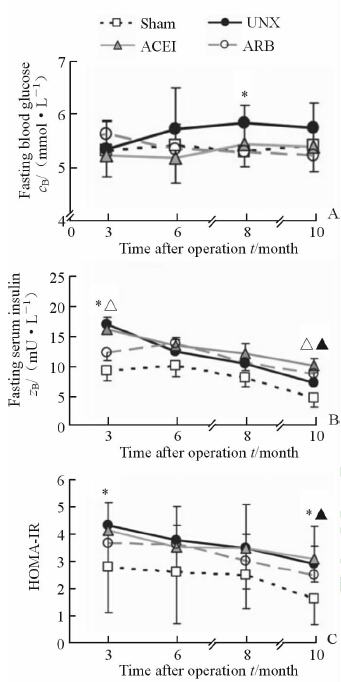
|
图 2 RAS阻滞剂对单肾切除大鼠糖代谢的影响 Fig 2 Effect of RAS blockade on glucose dysmetabolism in uninephrectomized rats A: Fasting blood glucose; B: Fasting serum insulin; C: HOMA-IR. *P<0.05,sham vs UNX; △P<0.05,UNX vs ACEI; ▲P<0.05,UNX vs ARB. n=10,x±s |
各组大鼠空腹胰岛素水平均随着时间延长有下降趋势(图 2B)。术后3个月时,UNX组胰岛素水平高于Sham组[(18.7±3.5) vs (12.1±2.4) mmol/L,P<0.05],ACEI治疗降低了单侧肾切除大鼠的高胰岛素水平[(15.6±2.7) mmol/L,P<0.05];术后10个月时,ACEI治疗组[(12.9±2.2) mmol/L]和ARB治疗组[(12.0±2.4) mmol/L]胰岛素水平高于UNX组[(11.4±1.9) mmol/L],差异有统计学意义(P<0.05)。
与空腹胰岛素水平的变化一致,4组大鼠HOMA-IR随手术时间延长而减小(图 2C)。术后3个月,UNX组HOMA-IR值高于Sham组(4.3±0.6 vs 2.8±0.3,P<0.05);术后10个月,UNX组HOMA-IR与Sham组差异有统计学意义(2.9±0.5 vs 1.6±0.2,P<0.05),ARB治疗能纠正单肾切除引起的胰岛素抵抗(UNX vs ARB: 2.9±0.5 vs 2.5±0.2,P<0.05)。 2.3 RAS阻滞剂对单肾切除大鼠残留肾AMPK表达的影响
蛋白免疫印迹结果(图 3)显示:UNX组大鼠残留肾皮质中AMPK表达明显少于Sham组,ACEI和ARB治疗均能使肾组织AMPK表达增强,差异有统计学意义(P<0.01)。
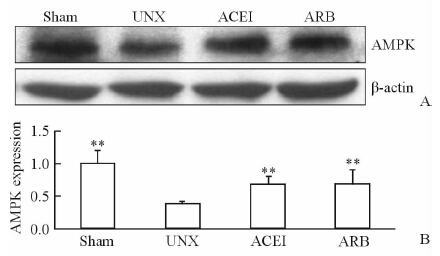
|
图 3 免疫印迹检测各组大鼠残留肾组织AMPK的表达 Fig 3 Expressions of AMPK in renal cortex by Western blotting analysis **P<0.01 vs UNX; n=10,x±s |
与蛋白免疫印迹结果一致,免疫荧光实验结果(图 4)表明:AMPK均匀一致地表达于Sham组大鼠肾小管上皮细胞内;UNX组大鼠肾皮质中AMPK表达减弱,除了代偿性结构功能相对正常的肾小管上皮细胞表达的AMPK接近于正常强度;ACEI和ARB治疗组AMPK表达水平整体上接近于Sham组,但不如Sham组均匀一致,个别肾小管表达强度类似于UNX组。

|
图 4 免疫荧光法检测各组大鼠残留肾组织AMPK的表达 Fig 4 Expressions of AMPK in renal cortex tissues by immunofluorescence A: Sham group; B: UNX group; C: ACEI group; D: ARB group. Original magnification: ×200 |
本研究结果表明单肾切除能引起大鼠肾功能损伤和糖代谢异常;ACEI或ARB治疗能纠正单肾切除引起的这些异常改变,包括肾皮质中AMPK的表达。结果证实单肾切除大鼠表现出的肾功能损伤伴糖代谢异常与大鼠RAS持续激活有关,且RAS通过减少肾皮质中AMPK的表达来干扰大鼠糖代谢。
RAS不仅是高血压和肾功能损伤的治疗靶点,也是代谢性疾病的重要影响因素[7]。在细胞水平,血管紧张素Ⅱ(Ang Ⅱ)通过增加氧化应激反应和改变胰岛素信号通路导致胰岛素抵抗,进而减少细胞葡萄糖的转运[8]。此外,Ang Ⅱ还能引起胰岛发生炎症,β细胞发生凋亡[8]。所以,单肾切除大鼠的糖代谢紊乱可能与Ang Ⅱ表达增强有关,这一观点已被证实[4]。单肾状态下,大鼠RAS激活不仅能引起残留肾损伤,还与胰岛中的转化生长因子β1激活有关[9, 10],后者可促进α-平滑肌肌动蛋白(α-smooth muscle actin,α-SMA)表达[11],α-SMA能引起成纤维细胞、血管平滑肌细胞等产生细胞外基质,最终引起纤维性损伤,如胰岛纤维化[4]。胰岛纤维组织逐渐取代正常分泌胰岛素的胰岛β细胞,导致胰岛素分泌减少,血糖升高。即便UNX大鼠年龄增加、胰岛纤维化引起胰岛素分泌不足,胰岛素抵抗仍使其空腹血胰岛素水平在各时间点均高于假手术组。所以,UNX大鼠空腹胰岛素水平由胰岛纤维化和胰岛素抵抗两个因素共同决定,且胰岛素抵抗引起的胰岛素含量改变大于胰岛纤维化。术后3个月时,UNX大鼠已出现了胰岛素抵抗,但胰岛纤维化不明显,所以UNX组空腹胰岛素水平高于假手术组;ACEI和ARB治疗均能在一定程度上改善胰岛素抵抗,但只有ACEI对空腹胰岛素的纠正有统计学意义(P<0.05)。术后10个月时,UNX大鼠仍存在胰岛素抵抗,但年龄增加和胰岛纤维化引起的胰岛素分泌不足越来越突出;RAS阻滞剂在手术后期可能对胰岛素分泌不足的纠正效果优于对胰岛素抵抗的纠正,所以RAS阻滞剂治疗组的空腹胰岛素水平高于UNX组。无论ACEI或ARB治疗组的空腹血糖、空腹胰岛素以及HOMA-IR的绝对值如何,与UNX组检测指标相比均有改善(P<0.05)。
除了直接影响大鼠糖代谢,Ang Ⅱ还可以通过改变残留肾中AMPK的表达来实施抗代谢稳定作用。AMPK在机体三大能量代谢平衡中起至关重要的调节作用。在糖代谢中,葡萄糖跨膜转运主要依赖于膜两侧葡萄糖的浓度梯度和葡糖糖转运蛋白的表达,尤其是葡萄糖转运体4(glucose transporters 4,GLUT4)[12],而AMPK能增加GLUT4的易位,促进葡萄糖吸收[13]。此外,AMPK通过使胰岛素受体底物1磷酸化来增强胰岛素的敏感性[14]。在糖代谢方面,Ang Ⅱ不仅完全对抗AMPK调节糖代谢的机制,如减少GLUT4的转位和影响胰岛素信号通路[15],而且还能阻止AMPK激活剂对糖代谢的调节作用[16]。因此,我们认为RAS引起的糖代谢紊乱还与肾皮质中AMPK的表达减少有关。本研究中UNX组大鼠肾皮质AMPK表达比Sham组明显减少,ACEI和ARB治疗均能纠正AMPK的表达并使其接近于正常水平。由此推断,RAS阻滞剂对单肾切除大鼠糖代谢异常的纠正,其机制和RAS阻滞剂对肾皮质中AMPK表达的恢复有关。
AMPK除了维持机体能量代谢的稳定,还对肾脏的生理功能有良好的调节作用[17, 18],如:调节肾小管运输,保护足细胞。当肾脏出现缺血、供能不足或者纤维性损伤时,AMPK激活剂表现出良好的治疗作用[19],且已应用于临床治疗[20]。因此,残留肾中AMPK表达减少不仅影响大鼠糖代谢,还影响大鼠肾功能。结合本研究结果和已证实的研究结论,我们认为单肾切除大鼠体内首先发生RAS持续激活,接着引起肾功能损伤和糖代谢紊乱,其作用机制与RAS抑制肾皮质中AMPK表达有关。因此,残留肾中Ang Ⅱ和AMPK的异常改变是肾功能损伤后糖代谢紊乱出现的重要原因。因此,RAS阻滞剂和AMPK激活剂联合使用可能为临床肾功能不全伴糖代谢异常患者提供新的治疗思路。
综上所述,本研究从肾病可能引起糖尿病的角度阐明了肾脏和代谢的相互关系。相关数据已经证实单肾切除不仅能引起糖代谢异常,还能导致脂肪代谢改变和残留肾癌变[2, 21]。因此,肾脏损伤和代谢疾病、癌症的发展息息相关,其潜在机制需要进一步研究,RAS和AMPK可能是其中的机制之一。
志谢:本研究实验场所和仪器由桂林医学院中心实验室提供,在此表示衷心的感谢!| [1] | Ahn J H, Yu J H, Ko S H, Kwon H S, Kim D J, Kim J H, et al. Prevalence and determinants of diabetic nephropathy in Korea: Korea national health and nutrition examination survey[J]. Diabetes Metab J, 2014, 38: 109-119. |
| [2] | Zhao H L, Sui Y, He L, Guan J, Xiao S J, Zhong D R, et al. Lipid partitioning after uninephrectomy[J]. Acta Diabetol,2011,48: 317-328. |
| [3] | Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey[J]. Lancet, 2012, 379: 815-822. |
| [4] | Sui Y, Zhao H L, Ma R C, Ho C S, Kong A P, Lai F M, et al. Pancreatic islet beta-cell deficit and glucose intolerance in rats with uninephrectomy[J]. Cell Mol Life Sci, 2007, 64:3119-3128. |
| [5] | Weir M R. The renoprotective effects of RAS inhibition: focus on prevention and treatment of chronic kidney disease[J]. Postgrad Med, 2009, 121: 96-103. |
| [6] | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects[J].Nature, 2003, 423: 762-769. |
| [7] | Mark L, Paragh G, Karadi I, Reiber I, Pados G, Kiss Z. How can we further improve the LDL-cholesterol target level achievement rate based on the Hungarian MULTI GAP 2011 study results and considering the new European dyslipidemia guidelines?[J] . Arch Med Sci, 2012, 8: 608-613. |
| [8] | Gandhi S, Srinivasan B P, Akarte A S. Effective blockade of RAAS by combination of aliskiren and olmesartan improves glucose homeostasis, glomerular filtration rate along with renal variables in streptozotocin induced diabetic rats[J].Eur J Pharm Sci, 2012, 46: 32-42. |
| [9] | Bechara R I, Pelaez A, Palacio A, Joshi P C, Hart C M, Brown L A, et al. Angiotensin Ⅱ mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung[J].Am J Physiol Lung Cell Mol Physiol, 2005, 289: L363-L370. |
| [10] | Huang Z, Jansson L, Sjoholm A. Pancreatic islet blood flow is selectively enhanced by captopril, irbesartan and pravastatin, and suppressed by palmitate[J].Biochem Biophys Res Commun, 2006, 346: 26-32. |
| [11] | Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts[J].J Cell Biol, 1993, 122: 103-111. |
| [12] | Steinberg G R, Kemp B E. AMPK in health and disease[J].Physiol Rev,2009,89:1025-1078. |
| [13] | Russell R R 3rd, Bergeron R, Shulman G I, Young L H. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR[J]. Am J Physiol, 1999, 277: H643-H649. |
| [14] | Jakobsen S N, Hardie D G, Morrice N, Tornqvist H E. 5’-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside[J]. J Biol Chem, 2001, 276: 46912-46916. |
| [15] | Yoshida D, Higashiura K, Shinshi Y, Satoh K, Hyakkoku M, Yoshida H, et al. Effects of angiotensin Ⅱ receptor blockade on glucose metabolism via AMP-activated protein kinase in insulin-resistant hypertensive rats[J]. J Am Soc Hypertens, 2009, 3: 3-8. |
| [16] | Shinshi Y, Higashiura K, Yoshida D, Togashi N, Yoshida H, Miyazaki Y, et al. Angiotensin Ⅱ inhibits glucose uptake of skeletal muscle via the adenosine monophosphate-activated protein kinase pathway[J]. J Am Soc Hypertens, 2007, 1: 251-255. |
| [17] | Fraser S A, Gimenez I, Cook N, Jennings I, Katerelos M, Katsis F, et al. Regulation of the renal-specific Na+-K+-2Cl-co-transporter NKCC2 by AMP-activated protein kinase (AMPK)[J]. Biochem J, 2007, 405: 85-93. |
| [18] | Alzamora R, Al-Bataineh M M, Liu W, Gong F, Li H, Thali R F, et al. AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney[J].Am J Physiol Renal Physiol,2013,305: F943-F956. |
| [19] | Seo-Mayer P W, Thulin G, Zhang L, Alves D S, Ardito T, Kashgarian M, et al. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia[J]. Am J Physiol Renal Physiol, 2011, 301: F1346-F1357. |
| [20] | Lempiainen J, Finckenberg P, Levijoki J, Mervaala E. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney[J]. Br J Pharmacol, 2012, 166: 1905-1915. |
| [21] | Yang X, Zhao H, Sui Y, Ma R C, So W Y, Ko G T, et al. Additive interaction between the renin-angiotensin system and lipid metabolism for cancer in type 2 diabetes[J].Diabetes, 2009, 58: 1518-1525. |
 2015, Vol. 36
2015, Vol. 36


