2. 第二军医大学基础医学部生理学教研室, 上海 200433
2. Department of Physiology, College of Basic Medical Sciences, Second Military Medical University, Shanghai 200433, China
女性绝经后发生动脉粥样硬化、脑卒中、冠心病等心血管疾病的风险明显升高[1, 2]。雌激素(E)具有改善由绝经引起心血管功能异常的作用,但其具体机制不明[3]。有研究证实,雌激素能够抑制血管紧张素Ⅱ(Ang Ⅱ)引起的大鼠心肌肥厚[4],而全身和器官局部肾素-血管紧张素系统(RAS)异常参与绝经后心血管功能异常的发生,如心脏局部RAS激活参与了心肌肥厚、心室重构和心衰的发生与发展[5]。研究证明,血管紧张素1型受体(AT1R)激活后通过G蛋白介导的信号通路促进绝经后心血管功能异常[6]。近年来研究发现,AT1R激活后通过β-arrestin2介导参与AT1R自身的脱敏和内化,从而发挥心血管保护作用[7]。TRV120027作为一种新型AT1R激动剂,能够使AT1R特异性地激活β-arrestin2介导而非G蛋白介导的信号通路。有文献证实,倾向β-arrestin2的AT1R激活可促进小鼠心肌损伤时心肌细胞的存活[8],改善心衰犬心脏负荷和降低全身和肾脏血管阻力[9],抑制由Ang Ⅱ诱导的大鼠心肌肥厚[10]。由此可见,研究倾向β-arrestin2的AT1R激活作为心血管疾病治疗新靶点意义重大。倾向β-arrestin2的AT1R激活是否参与雌激素改善由绝经引起心血管功能的调控国内外迄今少有报道。本研究通过明确β-arrestin2在雌激素改善卵巢切除(OVX)大鼠心脏中的表达情况变化,阐明倾向β-arrestin的AT1R激活在雌激素改善OVX大鼠心肌肥厚的作用意义,为雌激素改善OVX大鼠心血管功能的机制研究提供依据。
1 材料和方法 1.1 OVX模型建立健康雌性SD大鼠,体质量160~280 g,购自第二军医大学实验动物中心[动物生产许可证号SCXK(沪)-2013-0016]。随机选取SD雌性大鼠,采用氧气(流量为1 L/min)与异氟烷混合诱导麻醉成功后,以0.4 L/min维持麻醉。将大鼠固定,下腹部剪毛,消毒,进入腹腔,找到子宫,钝性分离并完整切除双侧卵巢,止血后逐层缝合、关腹,术后肌内注射青霉素2万U/kg。正常饲养6周后,OVX模型建立[11](OVX组)。只接受开腹分离卵巢而不切除卵巢的SD大鼠为假手术对照组(Sham组)。每组15只大鼠。
1.2 激素替代治疗OVX组及Sham组大鼠按照体质量大小依次编号后,采用随机数表法各组随机分出2个亚组,每个亚组5只大鼠。4组大鼠正常饲养2周后,OVX组及Sham组各分出的2个亚组:一个亚组每天按照30 mg/kg剂量颈部皮下给予雌激素(溶于生理盐水,购自Sigma公司),另一个亚组以生理盐水作为溶剂(Veh)对照。根据有无切除卵巢和雌激素替代治疗分别命名为: Sham+Veh组、Sham+E组、OVX+Veh组、OVX+E组。
1.3 样本采集雌激素替代治疗4周后,监测心血管指标,处死前从股静脉取血,8×g离心20 min,提取上清,放置于-80℃冰箱备用。处死后,称量心脏和子宫。心脏用液氮快速冷冻后,转移至-80℃保存。
1.4 心血管指标监测大鼠称体质量,2%戊巴比妥钠(0.3 mL/100 g)腹腔注射麻醉,将大鼠置于体温控制底板上,维持体温37℃行气管插管,于右侧股动脉插入PE动脉导管,连接至PowerLab/8SP(ADInstruments公司),监测平均动脉压(mean arterial pressure,MAP)和心率(heart rate,HR)。
1.5 血清雌激素水平测定雌二醇ELISA试剂盒购于BioTNT公司,按照说明书指导进行操作,测定各孔450 nm波长处的光密度值(D值),根据雌二醇标准品浓度与D值绘制标准曲线,计算雌激素的实际浓度。
1.6 β-arrestin和AT1R表达的检测将所取心脏标本剪取60~70 mg,液氮冷冻后研碎,按照每10 mg组织加入50 μL裂解液(由全细胞裂解液、蛋白酶抑制剂及磷酸酶抑制剂按100∶1∶1比例配制),提取细胞总蛋白,并测定各标本蛋白含量,加入上样缓冲液,煮沸100℃,变性5 min,行SDS-PAGE、转膜、5% BSA封闭。用Tris-盐酸缓冲液+Tween(TBST)洗膜3次,加入一抗(AT1R 抗体购自美国Epitomics公司,按1∶5 000稀释;β-arrestin1、β-arrestin2抗体均购自英国Abcam公司,按1∶1 000稀释),以GAPDH作为内参,室温孵育2 h,4℃过夜,TBST洗膜3次后,加入二抗,室温孵育1.5 h,TBST洗膜后进行化学发光显影,采用Gel-Pro Analyzer 1软件分析蛋白条带。
1.7 统计学处理采用GraphPad Prism 6.0软件进行统计分析,数据均以 x±s 表示,评价OVX模型数据采用t检验,其余各组比较采用双因素方差分析。检验水准(α)为0.05。
2 结 果 2.1 OVX模型的评价如表 1所示,卵巢切除后6周OVX大鼠体质量高于Sham组,子宫质量、子宫质量与体质量之比和血清雌激素水平低于Sham组(P<0.05)。
|
|
表 1 OVX模型与Sham组各项指标间比较 Tab 1 Comparison of parameters between OVX and Sham groups |
OVX模型大鼠饲养2周后,OVX大鼠给予Veh皮下注射4周后,心脏质量、体质量和两者之比高于Sham+Veh组(P<0.05);OVX大鼠雌激素替代治疗4周后,心脏质量和体质量较Veh对照组无明显下降,但两者之比下降[(2.847±0.166)‰ vs(3.763±0.433)‰,P<0.05]。与Sham+Veh组比,OVX+Veh的大鼠MAP[(140±6) mmHg vs(112±11) mmHg,1 mmHg=0.133 kPa;P<0.05]和HR[(457±28) min-1 vs (382±11) min-1,P<0.05]升高,而给予雌激素替代治疗后的OVX大鼠MAP[(115±6 ) mmHg vs (140±6 ) mmHg,P<0.05]和HR[(385±20) min-1 vs (457±28) min-1,P<0.05]不再升高。见表 2。
|
|
表 2 雌激素替代治疗后各组心血管指标比较 Tab 2 Comparison of cardiovascular function between OVX model group and estrogen replacement group |
图 1显示,β-arrestin1和AT1R蛋白在4组大鼠心脏中表达差异无统计学意义。而β-arrestin2蛋白水平,OVX+Veh组与Sham+Veh组比较明显降低(P<0.05),雌激素替代治疗4周后,能上调OVX大鼠β-arrestin2蛋白水平(P<0.05)。
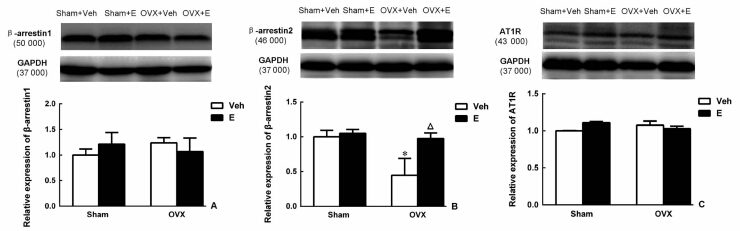
|
图 1 蛋白质印迹法检测各组大鼠心脏β-arrestin1 (A)、β-arrestin2 (B)和AT1R (C)蛋白表达 Fig 1 Comparison of β-arrestin1 (A),β-arrestin2 (B) and AT1R (C) protein in cardiac tissues in rats of different groups Veh: Vehicle; OVX: Ovariectomy; E: Estrogen;AT1R:Angiotensin receptor type 1. *P<0.05 vs Sham+Veh group; △P<0.05 vs OVX+Veh group. n=5, x±s |
大鼠OVX后造成手术绝经,大鼠心脏质量与体质量之比增加,MAP升高,HR增快,而雌激素替代治疗后上述指标均下降。研究证实,OVX大鼠可导致大鼠心肌肥厚[12],雌激素替代治疗可减轻由压力负荷增加导致的心肌肥厚[13],说明手术绝经大鼠心血管功能的改变可能与雌激素水平急剧下降有关[14]。另外,绝经心血管功能障碍可能与绝经导致的雌激素减少引起的RAS激活、交感神经活性增加、氧化应激增强有关[15, 16]。而假手术大鼠在卵巢能够分泌足够雌激素的情况下,不会产生因雌激素水平急剧下降而导致的心血管功能改变,因此,本研究中通过外源性补充的雌激素对假手术大鼠的心血管功能影响不大。
绝经后女性心血管疾病发病率明显增高,如冠状动脉粥样硬化、心肌肥厚和心肌收缩功能异常。研究证实雌激素替代治疗可改善心血管功能[17, 18],在抑制心肌肥厚方面可能与通过β-雌激素受体抑制钙依赖磷酸酶有关[4],另外,雌激素可能通过抑制炎症发挥心血管保护作用[19]。尽管针对雌激素改善心血管功能的机制研究较多,但其具体机制仍未明确。近年来,有文献报道卵巢切除后导致的心脏局部和全身循环中RAS的改变,如Ang Ⅱ水平的升高是导致心肌肥厚、心室重构和心肌收缩功能异常等心脏病变的重要机制之一[15, 20]。Ang Ⅱ作为RAS的主要成员,AT1R是介导Ang Ⅱ发挥心血管不良效应的主要受体,尽管卵巢切除后能引起心脏局部和全身循环中Ang Ⅱ升高,但卵巢切除后无论有无雌激素补充均对心脏AT1R的表达并无影响[5],这一点与本实验得到的结果一致。AT1R参与Ang Ⅱ的心血管不良效应主要有通过G蛋白介导的下游信号通路和通过介导AT1R自身脱敏和内化而发挥心血管保护作用的β-arrestin家族。在本实验中发现,无论有无雌激素补充,OVX后大鼠心脏β-arrestin1表达均无明显改变,而心脏β-arrestin2表达下调,并且雌激素替代治疗后可上调其表达。上述结果提示β-arrestin2可能参与了雌激素改善OVX大鼠心血管功能的效应。
本研究明确了OVX及雌激素替代治疗后心脏β-arrestin家族和AT1R表达水平的变化,提出了β-arrestin2介导的AT1R脱敏和内化可能参与了雌激素对OVX大鼠心血管功能的调控,为进一步明确雌激素改善OVX大鼠心血管功能的机制研究提供了依据,但仍存在不足之处:(1)缺乏对β-arrestin2的直接干预,无法观察通过抑制剂或基因敲除等方法直接下调β-arrestin2后雌激素对OVX大鼠的心血管保护效应是否存在。未能明确β-arrestin2通过介导的AT1R脱敏和内化在雌激素对OVX大鼠心血管保护效应中的具体作用。(2)雌激素发挥作用主要通过与雌激素受体结合起作用,而雌激素受体分为α-雌激素受体和β-雌激素受体2种[21]。另外,雌激素可通过基因途径和非基因途径发挥心血管保护效应,而雌激素调节β-arrestin2在心脏的表达主要同α-雌激素受体还是β-雌激素受体有关、经过基因途径还是非基因途径发挥作用需要进一步实验验证。(3)本研究主要针对心脏β-arrestin2对雌激素改善由绝经引起心血管功能的影响,而β-arrestin2在OVX大鼠心脏的变化趋势,是否在其他参与心血管功能调节的器官,如血管、肾脏和中枢神经系统中调控心血管功能的相关核团中存在同样的变化需要进一步研究。
尽管本研究和大量研究已经证实雌激素替代治疗能够减少绝经后女性心血管疾病的风险,但绝经后女性激素替代治疗仍然存在许多争议。如Rossouw等[22]发现绝经后女性激素替代治疗可增加卒中、乳腺癌、肺栓塞等风险,并认为对于绝经后女性激素替代治疗的整体疗效弊大于利。另外,针对绝经后女性雌激素替代治疗中雌激素用量也一直饱受争议,Campos等[23]证实在治疗大鼠绝经后引起的高血压低剂量雌激素跟高剂量雌激素效果一样;也有研究认为低剂量雌激素对绝经后女性运动后降压和降低交感神经活性的效应并无影响[24]。基于目前绝经后女性雌激素替代治疗存在的诸多争议,为绝经后女性雌激素替代治疗预防心血管疾病风险仍需进一步的研究来证实和评价。
| [1] | Bittner V. Menopause, age, and cardiovascular risk: a complex relationship[J]. J Am Coll Cardiol,2009,54:2374-2375. |
| [2] | Dessapt A L, Gourdy P. [Menopause and cardiovascular risk][J]. J Gynecol Obstet Biol Reprod (Paris),2012,41(7 Suppl):F13-F19. |
| [3] | Haines C J, Farrell E. Menopause management: a cardiovascular risk-based approach[J]. Climacteric,2010,13:328-339. |
| [4] | Pedram A,Razandi M,Lubahn D,Liu J,Vannan M, Levin E R. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin[J]. Endocrinology,2008,149:3361-3369. |
| [5] | Wang H,Jessup J A,Zhao Z,Da Silva J,Lin M,Macnamara L M,et al. Characterization of the cardiac renin angiotensin system in oophorectomized and estrogen-replete mRen2.Lewis rats[J]. PLoS One,2013,8:e76992. |
| [6] | Javeshghani D,Sairam M R,Neves M F,Schiffrin E L, Touyz R M. Angiotensin Ⅱ induces vascular dysfunction without exacerbating blood pressure elevation in a mouse model of menopause-associated hypertension[J]. J Hypertens,2006,24:1365-1373. |
| [7] | Tilley D G. Functional relevance of biased signaling at the angiotensin Ⅱ type 1 receptor[J]. Endocr Metab Immune Disord Drug Targets,2011,11:99-111. |
| [8] | Kim K S,Abraham D,Williams B,Violin J D,Mao L, Rockman H A. beta-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury[J]. Am J Physiol Heart Circ Physiol,2012,303:H1001-H1010. |
| [9] | Boerrigter G,Lark M W,Whalen E J,Soergel D G,Violin J D, Burnett J C Jr. Cardiorenal actions of TRV120027, a novel β-arrestin-biased ligand at the angiotensin Ⅱ type 1 receptor, in healthy and heart failure canines: a novel therapeutic strategy for acute heart failure[J]. Circ Heart Fail,2011,4:770-778. |
| [10] | Monasky M M,Taglieri D M,Henze M,Warren C M,Utter M S,Soergel D G,et al. The beta-arrestin-biased ligand TRV120023 inhibits angiotensin Ⅱ-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium[J]. Am J Physiol Heart Circ Physiol,2013,305:H856-H866. |
| [11] | Irigoyen M C,Paulini J,Flores L J,Flues K,Bertagnolli M,Moreira E D,et al. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats[J]. Hypertension,2005,46:998-1003. |
| [12] | Sharkey L C,Holycross B J,Park S,Shiry L J,Hoepf T M,McCune S A,et al. Effect of ovariectomy and estrogen replacement on cardiovascular disease in heart failure-prone SHHF/Mcc-facp rats[J]. J Mol Cell Cardiol,1999,31:1527-1537. |
| [13] | Cui Y H,Tan Z,Fu X D,Xiang Q L,Xu J W, Wang T H. 17 beta-estradiol attenuates pressure overload-induced myocardial hypertrophy through regulating caveolin-3 protein in ovariectomized female rats[J]. Mol Biol Rep,2011,38:4885-4892. |
| [14] | Yanes L L, Reckelhoff J F. Postmenopausal hypertension[J]. Am J Hypertens,2011,24:740-749. |
| [15] | Shenoy V,Grobe J L,Qi Y,Ferreira A J,Fraga-Silva R A,Collamat G,et al. 17beta-Estradiol modulates local cardiac renin-angiotensin system to prevent cardiac remodeling in the DOCA-salt model of hypertension in rats[J]. Peptides,2009,30:2309-2315. |
| [16] | Ikeno A,Minato H,Kohayakawa C, Tsuji J. Effect of OS-0544, a selective estrogen receptor modulator, on endothelial function and increased sympathetic activity in ovariectomized rats[J]. Vascul Pharmacol,2009,50:40-44. |
| [17] | Patterson E,Ma L,Szabo B,Robinson C P, Thadani U. Ovariectomy and estrogen-induced alterations in myocardial contractility in female rabbits: role of the L-type calcium channel[J]. J Pharmacol Exp Ther,1998,284:586-591. |
| [18] | van Eickels M,Grohé C,Cleutjens J P,Janssen B J,Wellens H J, Doevendans P A. 17beta-Estradiol attenuates the development of pressure-overload hypertrophy[J]. Circulation,2001,104:1419-1423. |
| [19] | Dantas A P, Sandberg K. Estrogen regulation of tumor necrosis factor-alpha: a missing link between menopause and cardiovascular risk in women?[J]. Hypertension,2005,46:21-22. |
| [20] | Ribeiro R F Jr,Pavan B M,Potratz F F,Fiorim J,Simoes M R,Dias F M,et al. Myocardial contractile dysfunction induced by ovariectomy requires AT1 receptor activation in female rats[J]. Cell Physiol Biochem,2012,30:1-12. |
| [21] | Chambliss K L,Wu Q,Oltmann S,Konaniah E S,Umetani M,Korach K S,et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice[J]. J Clin Invest,2010,120:2319-2330. |
| [22] | Rossouw J E,Anderson G L,Prentice R L,LaCroix A Z,Kooperberg C,Stefanick M L,et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial[J]. JAMA,2002,288:321-333. |
| [23] | Campos C,Sartorio C L,Casali K R,Fernandes R O,Llesuy S,da Rosa Araujo A S,et al. Low-dose estrogen is as effective as high-dose treatment in rats with postmenopausal hypertension[J]. J Cardiovasc Pharmacol,2014,63:144-151. |
| [24] | Oneda B,Forjaz C L,Bernardo F R,Araújo T G,Gusmāo J L,Labes E,et al. Low-dose estrogen therapy does not change postexercise hypotension, sympathetic nerve activity reduction, and vasodilation in healthy postmenopausal women[J]. Am J Physiol Heart Circ Physiol,2008,295:H1802-H1808. |
 2015, Vol. 36
2015, Vol. 36


