糖尿病(diabetes mellitus)是当前严重威胁人们健康的慢性疾病之一,据预测糖尿病患者数量在2030年将由2011年的3亿6600万人增长至5亿5200万人[1]。糖尿病视网膜病变(diabetic retinopathy,DR)是糖尿病最常见的微血管病变之一,常因黄斑水肿、视网膜脱离及玻璃体积血等造成视力减退甚至失明。虽然DR具体发病机制尚不明确,但世界两大糖尿病多中心试验研究DCCT[2]及UKPDS[3]均揭示高血糖是发生DR的基础因素,在高糖环境下视网膜氧化应激产物生成过量[4]、蛋白激酶C激活[5]、糖基化终末产物合成增多[6]等启动了视网膜组织细胞的损伤。本课题组设想从DR病变的基础——高糖环境着手,观察通过限制葡萄糖转运进入视网膜而降低视网膜局部含糖量是否可以缓解DR的进展。葡萄糖转运蛋白1(glucose transporter-1,GLUT1)是目前已知的葡萄糖通过血-视网膜屏障的唯一载体,其在视网膜中除分布于神经节细胞、光感受器细胞、Müller细胞等外主要表达于血-视网膜内屏障的血管内皮细胞和外屏障的视网膜色素上皮细胞[7]。我们拟利用siRNA靶向抑制视网膜GLUT1后,检测糖尿病小鼠视网膜炎症反应因子、视网膜血管白细胞黏滞数量和血-视网膜内屏障渗漏范围以比较微血管的病变程度,并据此探讨负性调节葡萄糖转运对DR的作用。 1 材料和方法 1.1 主要试剂和仪器
链脲佐菌素(streptozotocin,STZ)、焦碳酸二乙酯(diethyl pyrocarbonate,DEPC)、葡萄糖含量测定试剂盒购自美国Sigma-Aldrich公司,Western Blot电泳系统和蛋白质含量测定试剂盒购自美国Bio-Rad公司,LipofectamineTM RNAiMAX转染试剂购自美国Invitrogen公司,异硫氰酸荧光素-刀豆蛋白A(fluorescein isothiocyanate conjugated concanavalin A,FITC-ConA)、异硫氰酸荧光素-牛血清白蛋白(fluorescein isothiocyanate conjugated bovine serum albumin,FITC-BSA)购自美国Vector公司,兔抗小鼠GLUT1、细胞间黏附分子-1(intercellular cell adhesion molecule-1,ICAM-1)和肿瘤坏死因子α(tumor necrosis factor-α,TNF-α)一抗及羊抗兔二抗购自美国Millipore公司, 柠檬酸缓冲液和 磷酸盐缓冲液(phosphate buffer saline,PBS)购自北京天根生化科技有限公司,电子分析天平购自上海精科天平厂,光谱分析仪购自德国Spectro公司,倒置荧光显微镜购自日本Olympus株式会社,微量注射器购自美国Hamilton公司。 1.2 靶向GLUT1的siRNA合成
参考王耀东等[8]设计的有效siRNA序列,交由上海吉玛制药技术有限公司合成靶向GLUT1的siRNA,正义链5′-GGAATTCAATGCTGATGATGA-3′,反义链5′-TCATCATCAGCATTGAATTCC-3′,另合成非靶向性siRNA用作阴性对照,正义链5′-TTCTCCGAACGTGTCACGT-3′,反义链5′-ACGTGACACGTTCGGAGAA-3′。用DEPC处理过的生理盐水将siRNA溶解配制成浓度为20 μmol/L。 1.3 实验动物与分组
健康8周龄、无眼疾的近交系雄性C57BL/6小鼠 36只,体质量20~30 g,清洁级,购自南昌大学动物科学部,动物合格证号:赣动2012145。将小鼠打上耳钉编号后根据随机数字表随机选取,分为正常对照组、糖尿病对照组和 GLUT1siRNA组,每组各12只(24眼)。糖尿病对照组和GLUT1siRNA组小鼠禁食8 h后以50 mg/kg 腹腔注射STZ(注射前临时溶于pH 4.5的0.01 mol/L PBS),连续5 d;正常对照组予以pH 4.5的0.01 mol/L柠檬酸缓冲液。第7天采小鼠尾部静脉血,测量血糖(浓度大于3 g/L为糖尿病建模成功);建模成功后第21周再次测量血糖。分别于建模成功后第一天及第21周测各组小鼠体质量,并观察小鼠体型变化,记录饮水、进食和尿量。 1.4 玻璃体腔注射siRNA
建模成功后,对3组小鼠予以腹腔麻醉,眼部周围碘伏消毒,然后在显微镜下使用Hamilton 微量注射器于角膜缘外1 mm 向视神经方向进针,于瞳孔区见针尖后缓慢注射药物:GLUT1siRNA组于玻璃体腔内注入含20 μmol/L 靶向GLUT1的siRNA 1 μL以及转染试剂1 μL,正常对照组和糖尿病对照组则注入含20 μmol/L 非靶向性siRNA 1μL以及转染试剂1μL。以上操作每2周重复注射1次,共注射10次。注射结束后,即建模成功后21周时进行以下检测。 1.5 视网膜组织含糖量检测
3组小鼠各取6只,以脊椎脱臼法处死并取眼球,每组6只左眼球用作测量视网膜含糖量,6只右眼球用作后续免疫印迹检测。取视网膜组织,加入50 μL去离子水,加热至70~75 ℃(共15 min),然后超声裂解30 s,离心20 min,取上清液35 μL,加入165 μL葡萄糖含量测定试剂,并设立标准曲线和空白对照,应用光谱分析仪测定标本的吸光度,用SPECTRO STAR NANO MARS软件计算葡萄糖浓度。而后再取10 μL上清液,加入190 μL蛋白质含量测定试剂,设立标准曲线和空白对照,应用光谱分析仪测定标本的光密度,用SPECTRO STAR NANO MARS软件计算蛋白质浓度。以葡萄糖的量与蛋白质质量的比值表示视网膜葡萄糖含量(单位nmol/mg),计算公式为G×GV/[180.2×(P×PV)]。G:葡萄糖浓度(ng/mL),GV:葡萄糖含量测定反应液容积(mL),P: 蛋白质浓度(mg/mL),PV:蛋白质含量测定反应液容积(mL),葡萄糖相对分子质量为180.2。 1.6 免疫印迹检测
取方法1.5项中所述的3组小鼠的各6只右眼球,剥离视网膜组织,放入Eppendorf管,加入200 μL裂解液,超声裂解30 s,离心20 min,取上清液,测定样品蛋白质浓度。取等量蛋白样品沸水煮后加入SDS,进行聚丙烯酰胺凝胶电泳(PAGE),而后转膜90~120 min,分别加入GLUT-1、ICAM-1和TNF-α一抗,4℃过夜孵育,次日洗涤3遍后室温孵育二抗1 h,暗室中加入显影液,进行凝胶图像分析并测定条带灰度值。 1.7 视网膜血管白细胞黏滞检查
3组小鼠各取3只,分别予以氯胺酮(10 mg/kg)和赛拉嗪(60 mg/kg)混合液麻醉。剪开小鼠胸部皮肤及肋骨,暴露胸腔,夹闭降主动脉,剪开右心耳,将27G针头插入左心室,先用10 mL肝素(0.1 mg/mL) 与PBS混合液灌注冲洗未黏滞的白细胞,然后用20 μg/mL 的FITC-ConA(5 mg/kg)与PBS混合液标记黏滞的白细胞,再用10 mL PBS冲洗掉未与白细胞结合的FITC-ConA。以脊椎脱臼法处死小鼠,每组均取出6只眼球,直接放入4%多聚甲醛固定1 h,剥离视网膜,制备视网膜平铺片,使用荧光显微镜观察并进行计数。 1.8 血-视网膜内屏障渗漏
3组小鼠各取3只,分别予以氯胺酮(10 mg/kg)和赛拉嗪(60 mg/kg)混合液麻醉,经股静脉注射FITC-BSA(100 mg/kg),20 min后处死,每组均取出6只眼球,放入4%多聚甲醛固定30 min,剥离视网膜,制备视网膜平铺片,使用荧光显微镜观察渗漏情况。 1.9 统计学处理
采用SPSS 17.0统计软件进行统计分析。小鼠体质量、血糖水平、视网膜含糖量及免疫印迹检测灰度值等数据结果以 x±s 表示,组间比较采用ANOVA以及t检验,检验水准(α)=0.05。 2 结 果 2.1 糖尿病模型的建立以及3组小鼠体质量和血糖水平
糖尿病对照组和GLUT1siRNA组共24只小鼠均建模成功。3组小鼠在建模成功时体质量差异无统计学意义(P>0.05),第21周时正常对照组小鼠较其余两组糖尿病小鼠(糖尿病对照组和GLUT1siRNA组)分别重40.44%和35.59%,差异有统计学意义(P<0.01)。两组糖尿病小鼠体型均消瘦,饮水量、进食量和尿量均较正常对照组多(资料未列出)。血糖水平在刚建模成功时和第21周时正常对照组小鼠较糖尿病对照组低46.85%和55.37%,较GLUT1siRNA组低47.36%和54.39%,差异均有统计学意义(P<0.01),而糖尿病对照组和GLUT1siRNA组在两个测量时间点血糖水平差异均无统计学意义(表 1)。
|
|
表 1 3组小鼠体质量和血糖水平 Tab 1 Body weight and blood glucose levels of mice in three groups |
21周时正常对照组小鼠视网膜含糖量约为 (36.36±2.98) nmol/mg, 糖尿病对照组和GLUT1siRNA组小鼠视网膜含糖量分别高达 (156.73±8.01)nmol/mg和(78.44±4.96) nmol/mg,均高于正常对照组(P<0.01),但GLUT1siRNA组小鼠的视网膜含糖量低于糖尿病对照组,为后者的50.05%(P<0.01),见图 1。
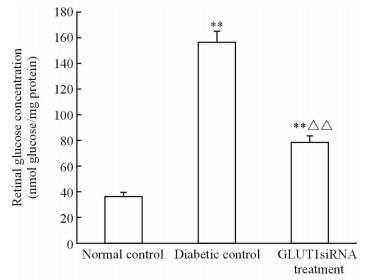 | 图 1 3组小鼠视网膜的含糖量测定 Fig 1 Retinal glucose concentrations of mice in three groups n=6,x±s . **P<0.01 vs normal control; △△P<0.01 vs diabetic control |
我们应用免疫印迹法检测GLUT1在小鼠神经视网膜层中的表达后进行统计学分析发现,GLUT1在糖尿病条件下表达上调,但使用靶向GLUT1的siRNA处理后小鼠视网膜GLUT1表达下调,较正常对照组表达约下降77.00%,仅为糖尿病对照组的8.07%,差异有统计学意义(P<0.01,图 2)。
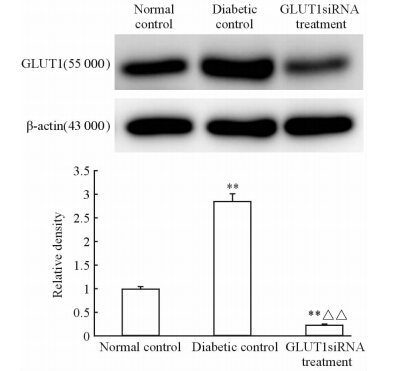 | 图 2 GLUT1在3组小鼠神经视网膜层中的表达 Fig 2 GLUT1 expression in neural retina of mice in three groups n=6,x±s . **P<0.01 vs normal control; △△P<0.01 vs diabetic control |
我们同时也检测了视网膜色素上皮层上GLUT1的表达,与神经视网膜层结果不同,GLUT1在GLUT1siRNA组仅为糖尿病对照组的50.22%,差异有统计学意义(P<0.01),但与正常对照组间差异无统计学意义(P>0.05),见图 3。
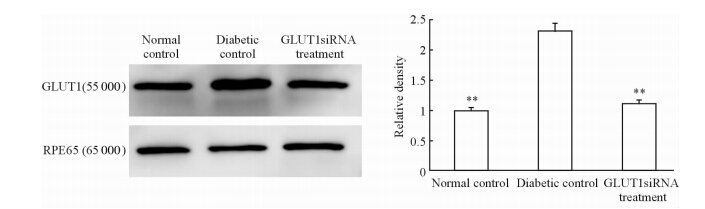 | 图 3 GLUT1在3组小鼠视网膜色素上皮层中的表达 Fig 3 GLUT1 expression in retinal pigment epithelium of mice in three groups n=6,x±s . **P<0.01 vs diabetic control |
视网膜血管白细胞黏滞度是视网膜炎症反应的一个重要指标[9],也是DR的早期病理改变。我们发现在正常对照组中视网膜血管基本没有白细胞黏滞(图 4A),糖尿病对照组(图 4B)和GLUT1siRNA组(图 4C)的小鼠均出现较多的白细胞黏滞,但GLUT1siRNA组其黏滞的白细胞数量经计数约为糖尿病对照组总数的52.76%,差异有统计学意义(P<0.01,图 4D)。
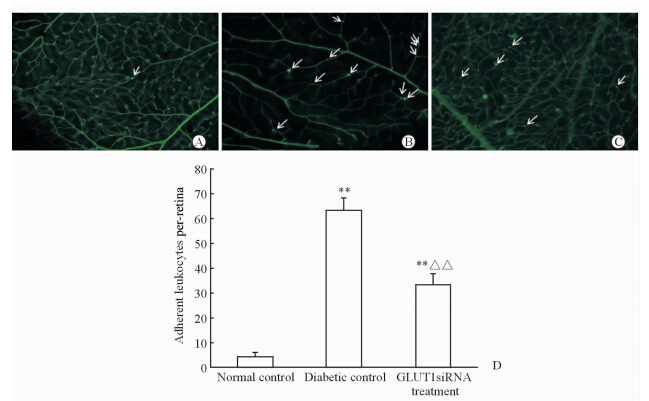 | 图 4 3组小鼠视网膜血管白细胞黏滞度 Fig 4 Leukostasis assay of retinal blood vessels of mice in three groups A: Normal control; B: Diabetic control; C: GLUT1siRNA treatment; D: Statistical analysis,n=6,x±s . **P<0.01 vs normal control,△△P<0.01 vs diabetic control. Fluorescence microscope. Original magnification: ×400(A-C). White arrows (A-C) indicate leukocytes adhering to retinal vasculatures |
国内外研究已证实DR是一种慢性炎症性疾病[10],ICAM-1和TNF-α是两个重要的炎症反应标记物。应用免疫印迹法检测ICAM-1和TNF-α,我们发现ICAM-1在糖尿病对照组和GLUT1siRNA组中表达均较正常对照组上调,差异均有统计学意义(P<0.01),但GLUT1siRNA组小鼠视网膜ICAM-1表达约为糖尿病对照组的66.14%,差异有统计学意义(P<0.05),见图 5。
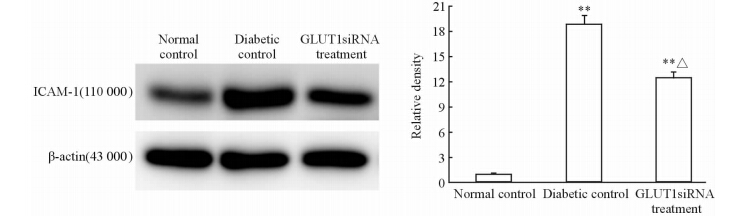 | 图 5 3组小鼠视网膜炎症因子ICAM-1的表达 Fig 5 Inflammation factor ICAM-1 expression in retina of mice in three groups ICAM-1:Intercellular cell adhesion molecule-1. n=6,x±s . **P<0.01 vs normal control; △P<0.05 vs diabetic control |
TNF-α检测也取得类似的结果,其在糖尿病对照组和GLUT1siRNA组中表达同样均较正常对照组上调,差异均有统计学意义(P<0.01),但GLUT1siRNA组小鼠视网膜TNF-α表达约为糖尿病对照组的54.76%,差异有统计学意义(P<0.01),见图 6。
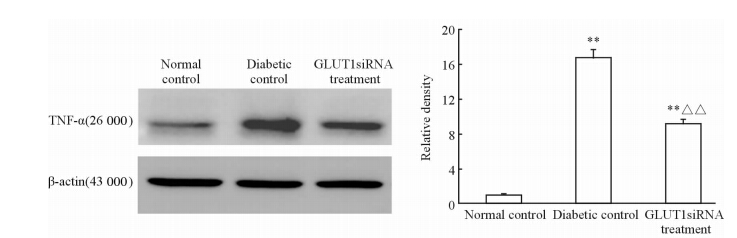 | 图 6 3组小鼠视网膜炎症因子TNF-α的表达 Fig 6 Inflammation factor TNF-α expression in retina of mice in three groups TNF-α:Tumor necrosis factor-α. n=6,x±s . **P<0.01 vs normal control; △△P<0.01 vs diabetic control |
我们使用荧光显微镜通过观察和比较FITC-BSA以反映血-视网膜内屏障的渗漏情况。结果发现在正常对照组中血-视网膜内屏障完整,未发现荧光渗漏,糖尿病对照组和GLUT1siRNA组的小鼠均出现了荧光渗漏区域,但GLUT1siRNA组其荧光渗漏区域较糖尿病对照组数量少,且渗漏面积也较小(图 7)。
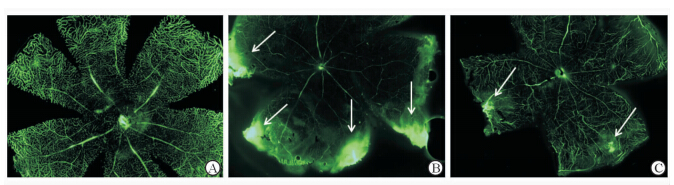 | 图 7 血-视网膜内屏障破坏造成的荧光素渗漏 Fig 7 Inner blood-retina barrier breakdown induced fluorescence leakage A: Normal control; B: Diabetic control; C: GLUT1siRNA treatment. White arrows indicate for fluorescence leakage areas. Original magnification: ×400 |
DR是糖尿病最常见和严重的眼部并发症,其具体发病机制目前尚不明确,大型随机对照临床研究DCCT[2]及UKPDS[3]已证实高血糖在DR及其他糖尿病并发症中的重要作用,高糖对视网膜细胞的作用机制可能包括影响特定基因的表达、使糖基化终末产物增多以及增加氧化应激反应[11]。既然糖尿病条件下视网膜组织所处的高糖微环境对其有损伤效应,有理由推测控制视网膜组织局部含糖量、逆转高糖环境可能是解决此问题的一把钥匙,视网膜的葡萄糖由血液转运而来,然而葡萄糖由于其水溶性而无法通过哺乳动物细胞膜的磷脂双分子层,因此视网膜组织细胞摄取葡萄糖需依靠一类转运葡萄糖的载体蛋白即GLUT[12],其中 GLUT1是葡萄糖通过血-视网膜屏障的唯一载体[7]。Kumagai等[13]应用免疫细胞化学法定量研究糖尿病患者眼球(没有或仅有轻微视网膜病变)中GLUT1的表达,发现半数以上的眼球视网膜GLUT1活性较正常对照组高出18倍,提示GLUT1上调可通过运输大量葡萄糖进入视网膜而介导细胞损伤。
siRNA是一类19~21 bp大小的RNA片段,可特异性降解某个特定基因mRNA,从而对该基因产生抑制作用。本实验参考既往研究有效的GLUT1siRNA序列并进行局部应用以观察其是否可以减少葡萄糖转运进入视网膜。如前所述,尽管两组糖尿病小鼠的总体血糖水平在建模后第20周差异无统计学意义,然而GLUT1siRNA组在接受玻璃体腔注射GLUT1siRNA后,视网膜组织中GLUT1表达相应下调,较糖尿病对照组下降约91.93%,较正常对照组约下降77.00%。我们同时测得GLUT1siRNA组视网膜局部含糖量仅为糖尿病对照组的50.05%,说明GLUT1siRNA抑制GLUT1后确实可以有效减少葡萄糖转运进入视网膜。但是我们也发现GLUT1siRNA组视网膜局部含糖量仍然较正常对照组高约53.64%,其原因在于我们使用玻璃体腔法注射GLUT1siRNA对视网膜内屏障的GLUT1有明显的抑制作用,然而对视网膜色素上皮层即视网膜外屏障作用有限,视网膜色素上皮层GLUT1表达未出现明显下调,同时由于糖尿病条件下GLUT1其生物学活性较正常条件下上调[14],因此从视网膜外屏障转运进入的葡萄糖导致GLUT1siRNA组视网膜含糖量较正常对照组高。但我们通过限制内屏障GLUT1也已达到了预先设想的条件,接下来我们通过观察炎症反应因子、视网膜血管白细胞黏滞数量和血-视网膜内屏障渗漏范围的变化探讨了局部含糖量减少是否可以影响微血管病变程度。
炎症反应是DR微血管病变的重要环节,大量研究发现糖尿病动物模型中视网膜白细胞数量增多,黏附能力增强,变形能力下降[15,16]。由于DR患者白细胞通过比自身直径小的毛细血管时被动变形能力下降,导致白细胞黏附率增加,随着DR的病情进展,白细胞黏附率增加更为明显[17]。因此我们使用视网膜血管白细胞黏滞检查分析DR炎症反应程度,发现GLUT1siRNA组小鼠发生黏滞的白细胞数量尽管比正常对照组多,但仅为糖尿病对照组总数的52.76%。我们同时测定了两种炎症反应标记物:趋化因子ICAM-1和细胞因子TNF-α的水平,发现这两种炎症因子在GLUT1siRNA组小鼠视网膜中的表达分别仅为糖尿病对照组的66.14%和54.76%,提示GLUT1siRNA限制葡萄糖转运进入视网膜后糖尿病小鼠炎症反应相对较轻。ICAM-1和其配体CD18在介导白细胞黏滞中发挥了重要作用[18],抑制ICAM-1可以显著减轻白细胞黏滞和血管通透性[19],TNF-α同样在DR视网膜中表达上调[20]。血-视网膜屏障的破坏是视网膜水肿尤其是黄斑水肿发生的重要原因,其原因可能是白细胞黏滞的增加和炎症因子表达上调[10]。如前所述,我们发现在GLUT1siRNA组白细胞黏滞和炎症因子均较糖尿病对照组轻,进一步通过血-视网膜内屏障渗漏检查发现其渗漏区域也较糖尿病对照组数量少,且渗漏面积也较小,说明视网膜的相对低糖环境对血-视网膜内屏障产生了保护作用。
综上所述,应用GLUT1siRNA局部注射抑制视网膜GLUT1后,糖尿病小鼠的视网膜含糖量下降,从而形成了一个视网膜相对低糖环境。在此环境下视网膜微血管病变较普通糖尿病小鼠有所缓解。结果提示,通过竞争性抑制GLUT1从而限制视网膜局部含糖量有可能成为未来防治DR的一个新方向。
4 利益冲突
所有作者声明本文不涉及任何利益冲突。
| [1] | Feldmann H, Geisbert T W. Ebola haemorrhagic fever [J]. Lancet, 2011, 377:849-862. |
| [2] | Sanchez A, Geisbert T W, Feldmann H. Filoviridae: Marburg and Ebola viruses[M]//Fields B N, Knipe D M, Howley P M. Fields virology. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2007:1410-1448. |
| [3] | Colebunders R, Borchert M.Ebola haemorrhagic fever-a review [J]. J Infect, 2000, 40:16-20. |
| [4] | WHO. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International Study Team[J]. Bull World Health Organ, 1978, 56: 247-270. |
| [5] | Towner J S, Sealy T K, Khristova M L, Albariño C G, Conlan S, Reeder S A, et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda[J]. PLoS Pathog, 2008, 4:e1000212. |
| [6] | Jahrling P B, Geisbert T W, Dalgard D W, Johnson E D, Ksiazek T G, Hall W C, et al. Preliminary report: isolation of Ebola virus from monkeys imported to USA Towner [J]. Lancet, 1990, 335: 502-505. |
| [7] | Groseth A, Feldmann H, Strong J E. The ecology of Ebola virus [J].Trends Microbiol, 2007, 15:408-416. |
| [8] | Wilson J A, Bosio C M, Hart M K. Ebola virus: the search for vaccines and treatments [J]. Cell Mol Life Sci, 2001, 58(12-13):1826-1241. |
| [9] | Feldmann H, Wahl-Jensen V, Jones S M, Ströher U. Ebola virus ecology: a continuing mystery[J]. Trends Microbiol, 2004, 12:433-437. |
| [10] | Ebola virus disease[R]. http://www.who.int/mediacentre/factsheets/fs103/en/ (2014-09)[2015-01-22] |
| [11] | Kiley M P, Bowen E T, Eddy G A, Isaäcson M, Johnson K M, McCormick J B, et al. Filoviridae: a taxonomic home for Marburg and Ebola viruses? [J]. Intervirology, 1982, 18(1-2):24-32. |
| [12] | Cook J D, Lee J E. The secret life of viral entry glycoproteins: moonlighting in immune evasion [J]. PLoS Pathog, 2013, 9:e1003258. |
| [13] | Schoepp R J, Rossi C A, Khan S H, Goba A, Fair J N. Undiagnosed acute viral febrile illnesses, Sierra Leone [J]. Emerg Infect Dis, 2014, 20:1176-1182. |
| [14] | Nakayama E, Saijo M. Animal models for Ebola and Marburg virus infections[J]. Front Microbiol, 2013, 4:267. |
| [15] | Dowell S F, Mukunu R, Ksiazek T G, Khan A S, Rollin P E, Peters C J. Transmission of Ebola haemorrhagic fever: a study of risk factors in family members, Kikwit, Democratic Republic of the Congo, 1995. Commission de Lutte contre les Epidémies à Kikwit[J]. J Infect Dis, 1999, 179(Suppl):S87-S91. |
| [16] | Geisbert T W, Hensley L E, Larsen T, Young H A, Reed D S, Geisbert J B, et al. Pathogenesis of Ebola haemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection[J]. Am J Pathol, 2003, 163:2347-2370. |
| [17] | Casillas A M, Nyamathi A M, Sosa A, Wilder C L, Sands H. A current review of Ebola virus: pathogenesis, clinical presentation, and diagnostic assessment [J]. Biol Res Nurs, 2003, 4:268-275. |
| [18] | Geisbert T W, Young H A, Jahrling P B, Davis K J, Larsen T, Kagan E, et al. Pathogenesis of Ebola haemorrhagic fever in primate models: evidence that haemorrhage is not a direct effect of virus induced cytolysis of endothelial cells[J]. Am J Pathol, 2003, 163:2371-2382. |
| [19] | Paessler S, Walker D H. Pathogenesis of the viral hemorrhagic fevers [J]. Annu Rev Pathol, 2013, 8:411-440. |
| [20] | Formenty P, Leroy E M, Epelboin A, Libama F, Lenzi M, Sudeck H, et al. Detection of Ebola virus in oral fluid specimens during outbreaks of Ebola virus hemorrhagic fever in the Republic of Congo[J]. Clin Infect Dis, 2006, 42:1521-1526. |
| [21] | Towner J S, Rollin P E, Bausch D G, Sanchez A, Crary S M, Vincent M, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome[J]. J Virol, 2004, 78:4330-4341. |
| [22] | Zaki S R, Shieh W J, Greer P W, Goldsmith C S, Ferebee T, Katshitshi J, et al. A novel immunohistochemical assay for the detection of Ebola virus in skin: implications for diagnosis, spread, and surveillance of Ebola hemorrhagic fever. Commission de Lutte contre les Epidémies à Kikwit [J]. J Infect Dis, 1999, 179 (Suppl 1):S36-S47. |
| [23] | Ksiazek T G, Rollin P E, Williams A J, Bressler D S, Martin M L, Swanepoel R, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995[J]. J Infect Dis, 1999, 179 (Suppl 1):S177-S187. |
| [24] | Pushko P, Bray M, Ludwig G V, Parker M, Schmaljohn A, Sanchez A, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus [J]. Vaccine, 2000, 19:142-153. |
| [25] | Phoolcharoen W, Dye J M, Kilbourne J, Piensook K, Pratt W D, Arntzen C J, et al. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge[J]. Proc Natl Acad Sci USA, 2011, 108:20695-20700. |
| [26] | Warfield K L, Swenson D L, Olinger G G, Kalina W V, Aman M J, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge[J]. J Infect Dis, 2007, 196(Suppl 2):S430-S437. |
| [27] | Halfmann P, Ebihara H, Marzi A, Hatta Y, Watanabe S, Suresh M, et al. Replication-deficient ebolavirus as a vaccine candidate[J]. J Virol, 2009, 83:3810-3815. |
| [28] | Sullivan N J, Geisbert T W, Geisbert J B, Xu L, Yang Z Y, Roe-derer M, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates[J]. Nature, 2003, 424:681-684. |
| [29] | Geisbert T W, Bailey M, Hensley L, Asiedu C, Geisbert J, Stanley D, et al. Recombinant adeno-virus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against Ebola virus challenge[J]. J Virol, 2011, 85:4222-4233. |
| [30] | Richardson J S, Yao M K, Tran K N, Croyle M A, Strong J E, Feldmann H, et al. Enhanced protection against Ebola virus mediated by an improved adenovirus-based vaccine[J]. PLoS One, 2009, 4:e5308. |
| [31] | Sridhar S. Ebola and Marburg vaccines for Africa: one step closer[J]. Lancet, 2014, 22, pii: S0140-6736(14)62445-4. |
| [32] | Feldmann H, Jones S M, Daddario-Dicaprio K M, Geisbert J B, Stro her U, Grolla A, et al. Effective post-exposure treatment of Ebola infection[J]. PLoS Pathog, 2007, 3:e2. |
| [33] | Geisbert T W, Daddario-DiCaprio K M, Williams K J, Geisbert J B, Leung A, Feldmann F, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates[J]. J Virol, 2008, 82:5664-5668. |
| [34] | Bukreyev A, Yang L, Zaki S R, Shieh W J, Rollin P E, Murphy B R, et al. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge[J]. J Virol, 2006, 80:2267-2279. |
| [35] | Rubin E J, Baden L R. Out of Africa—caring for patients with Ebola[J]. N Engl J Med, 2014, 371:2430-2432. |
 2015, Vol. 36
2015, Vol. 36


