2. 第二军医大学长海医院口腔科, 上海 200433
2. Department of Stomatology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
头颈部鳞状细胞癌(head and neck squamous cell carcinoma,HNSCC)是一种非均质癌瘤,好发于口腔、咽部和喉部,其发病率和病死率都较高,5年总体生存率仍维持在50%~60%[1]。目前的研究表明,HNSCC的发生与感染人乳头状瘤病毒、吸烟和饮酒等因素有明显相关性[2],但其发生、发展的分子机制尚不明确。
MicroRNAs(miRNAs)是一组广泛存在于真核生物中非编码的19到24个核苷酸长度的RNA,在翻译水平调控基因的表达[3]。miRNAs在个体发育、病毒感染及肿瘤的发病机制中均具有重要作用[4, 5]。研究表明,miRNAs 在肿瘤的发病机制中扮演了重要角色,大量的miRNAs起着促癌或抑癌作用[6]。
目前,已有多个miRNAs被证明在HNSCC的发生、发展中起着重要作用。如miR-99a在HNSCC中呈低表达,而过表达miR-99a则能抑制细胞增殖和诱导凋亡[7];miR-375的表达下调与HNSCC的不良预后有相关性[8];miR-133a则参与了HNSCC的侵袭和迁移等[9]。
miR-141在胰腺癌、卵巢癌和胃癌中能抑制肿瘤的生长和迁移[10, 11, 12],但miR-141在HNSCC的作用目前却未见报道。本研究希望通过上调或抑制miR-141的表达水平来探究其在HNSCC发病机制中的作用,以期为抗肿瘤药物的研发提供可能的靶点。
1 材料和方法 1.1 标本收集2010年1月至2013年12月于第二军医大学长征医院手术切除并经病理确诊的HNSCC 19例,其中喉癌11例、口腔鳞状细胞癌8例;男性15例、女性4例,平均年龄(42±8)岁;所有患者术前均未接受放化疗。每例均取癌及癌旁正常组织(距癌组织边缘>2 cm,病理证实无癌细胞)各1块(约1.0 cm×0.5 cm),-80℃保存。本研究经第二军医大学长征医院伦理委员会审批通过并备案,所有患者均签署知情同意书。
1.2 细胞培养HEK293、人喉癌细胞株(Hep-2)和人舌鳞状细胞癌细胞株(SCC-9)均购自中国科学院上海细胞库。细胞株置于含10% 胎牛血清的DMEM培养液中培养,并加入2 mmol/L 左旋谷酰胺以及100 μg/mL青霉素[生工生物工程(上海)股份有限公司]。具体培养方法参照Wu等[13, 14]的方法。
1.3 荧光实时定量PCR检测miR-141的表达miR-141的表达使用mirVanaTM qRT-PCR miRNA Detection Kit(Ambion,美国),该引物由上海生物工程技术服务有限公司设计并验证。miR-141顺义链:5′-CGC CAG GAT AAA TTG ACG CAC CAT CTT TAC-3′;反义链:5′-CCG CCT TAA CAC TGT CTG GTA ATC GCC AGG ATA AAT TGA CGC A-3′; 阴性对照顺义链:5′-UUC UCC GAA CGU GUC ACG UTT-3′;反义链:5′-ACG UGA CAC GUU CGG AGA ATT-3′;内参miR-U6:5′-AUA UGG AAC GCU UCA CGA AUU-3′。在定量过程中以同一例患者的癌旁正常组织作为对照,并计算癌组织标本miR-141的相对表达水平。
1.4 MTT检测Hep-2和SCC-9细胞增殖细胞以5×103个/孔的密度接种于96孔板,经5 d培养后,用MTT法检测细胞增殖情况,光密度(D)值均由酶标仪读出。
1.5 miRNA mimics、miRNA反义寡核苷酸(ASO)转染miR-141 mimics以及miR-141 ASO购于GenePharma(中国),miRNAs mimics、miRNA ASO以及阴性对照使用Lipofectamine 2000(Invitrogen,加拿大)进行转染,转染后48 h的细胞用于实验[15]。
1.6 生物信息学方法预测miR-141的靶基因采用TargetScanHuman(http://www.targetscan.org/vert_61/)预测miR-144的靶基因。
1.7 统计学处理统计分析使用SPSS 17.0软件。数据以 x±s 形式表示,当两组的平均值比较时使用双尾Student’s t检验,配对标本中miR-141水平的比较用秩和检验。检验水准(α)为0.05。
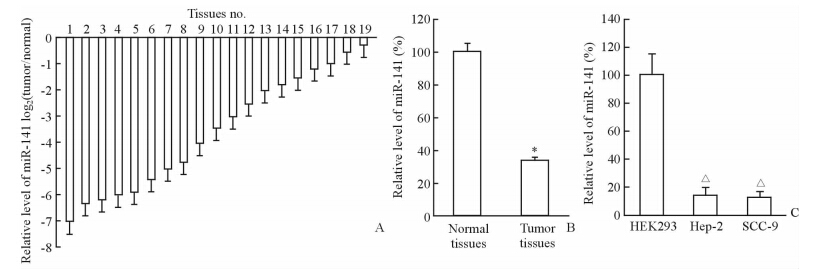
2 结 果 2.1 HNSCC组织和细胞中miR-141的表达水平通过qRT-PCR分析19例HNSCC组织标本miR-141的表达水平,结果显示,19例HNSCC肿瘤组织中的miR-141水平均低于相应的癌旁正常组织(图1A)。统计结果表明:19例HNSCC组织标本中miR-141的平均值低于癌旁正常组织中的miR-141的平均值,HNSCC组织中的miR-141的平均表达量约为正常组织中的37.4%(图1B,P<0.05)。相对于HEK293细胞,miR-141在Hep-2和SCC-9细胞中均呈低水平表达(图1C,P < 0.05)。
|
图 1 miR-141在头颈鳞状细胞癌(HNSCC)组织和细胞中的表达水平 Fig 1 Expression of miR-141 in head and neck squamous cell carcinoma(HNSCC)tissues and cells A: The relative expression of miR-141 in 19 HNSCC tissues and the matched tumor-adjacent normal tissues; B: The mean level of miR-141 in HNSCC tissues and the matched tumor-adjacent normal tissues; C: The miR-141 expressions in Hep-2,SCC-9 and HEK293 cells were assayed by qRT-PCR analysis. *P<0.05 vs normal tissues; △P<0.05 vs HEK293 cells group. n=3 in A,n=19 in B,n=3 in C; x±s |
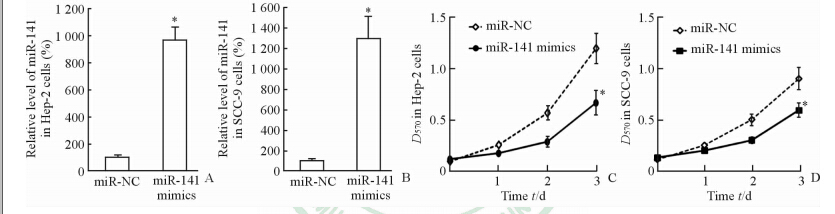
用miR-141 mimics转染Hep-2和SCC-9细胞48 h后,Hep-2和SCC-9细胞中miR-141的表达水平上调(图2A、2B)。MTT检测细胞增殖实验结果显示,上调miR-141表达3 d时能抑制Hep-2和SCC-9的细胞增殖(P<0.05,图2C、2D)。
|
图 2 转染miR-141 mimics后促进Hep-2和SCC-9细胞中miR-141表达及抑制Hep-2和SCC-9细胞增殖 Fig 2 Transfection of miR-141 mimics enhanced the miR-141 expression in Hep-2 and SCC-9 cells and inhibited the proliferation of Hep-2 and SCC-9 cells A,B: 48 h after miR-141 mimics transfection,the miR-141 expressions in Hep-2 and SCC-9 cells were assayed by qRT-PCR analysis; C,D: The cell proliferation was assayed by MTT analysis at the indicated time points. miR-NC: Negative control miRNA. *P<0.05 vs miR-NC group. n=3, x±s |
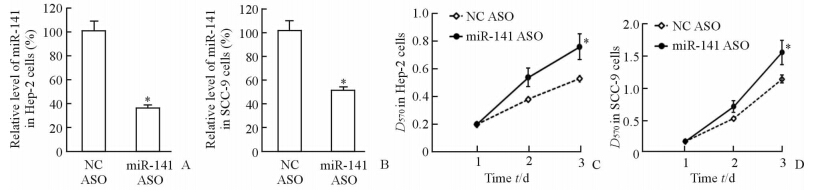
通过转染miR-141 ASO 抑制miR-141的表达,结果表明转染miR-141 ASO 48 h后,在Hep-2和SCC-9细胞中,miR-141的表达水平下调(图3A、3B)。MTT检测细胞增殖实验结果表明,抑制miR-141的表达3 d能促进Hep-2和SCC-9细胞的增殖(P<0.05,图3C、3D)。
|
图 3 转染miR-141 ASO后抑制Hep-2和SCC-9细胞中miR-141表达及促进Hep-2和SCC-9细胞增殖 Fig 3 Transfection of miR-141 ASO down-regulated the miR-141 expressions in Hep-2 and SCC-9 cells and promoted the proliferation of Hep-2 and SCC-9 cells A,B: 48 h after miR-141 ASO transfection,the miR-141 expressions in Hep-2 and SCC-9 cells were assayed by qRT-PCR analysis,the miR-141 expression in normal tissue was arbitrarily defined as 100%; C,D: The cell proliferation was assayed by MTT analysis at the indicated time points. ASO: Antisense oligonucleotide; NC ASO: Negative control miRNA ASO. P<0.05 vs NC ASO group. n=3,x±s |
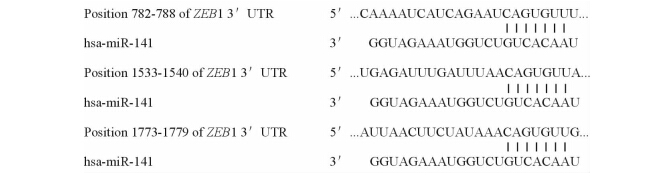
通过生物信息学算法发现,miR-141能够与ZEB1 3′UTR的3个位点结合(图4)。据此,我们推测miR-141可能通过ZEB1抑制HNSCC的生长。
|
图 4 ZEB1基因与miR-141的3个结合位点 Fig 4 Putative targeted genes of miR-141 were predicted by Target Scan Human and the three binding sites |
肿瘤是一类复杂的基因突变疾病。在肿瘤中,由于主要信号通路的缺失或改变,失控的细胞失去自然有序的稳态。绝大多数的 HNSCC 发生于口腔、口咽、喉咽以及喉部。尽管目前在手术、放化疗方面取得了进展,但 HNSCC 晚期患者的5年生存率只有50%~60%[1]。目前,已有越来越多证据表明 miRNAs 在多种人类肿瘤细胞中异常表达,并且参与肿瘤细胞的增殖、分化及凋亡等,其在人类肿瘤中的基因突变、缺失或异常表达已成为研究的热点之一[6, 7, 8, 9]。
近期的研究发现miR-141在多种肿瘤的发生、发展中起到重要作用,如miR-141在胰腺癌中通过MAP4K4抑制细胞增殖和侵袭[16]; miR-141可作为判断卵巢癌预后的重要指标[17]; miR-141能抑制胃癌的生长和迁移[18]。但miR-141在HNSCC发生、发展中的作用一直未见报道。本研究表明,HNSCC组织的miR-141表达水平低于癌旁正常组织; 上调miR-141的表达能够抑制Hep-2和SCC-9细胞的增殖,而抑制miR-141的表达可以促进Hep-2和SCC-9细胞的增殖。
通过生物信息学算法发现,miR-141能够与ZEB1 3′UTR的3个位点结合。而有研究表明,ZEB1基因能促进肿瘤发生发展[19]。因此,我们认为miR-141可能通过ZEB1基因,抑制HNSCC的生长。要证实这一点,我们计划在下一步的研究中,首先分析ZEB1 mRNA或蛋白在HNSCC 的表达水平,并考察ZEB1的表达水平与miR-141的表达水平是否具有相关性,然后通过双荧光报告基因的方法证实miR-141是否能靶向抑制ZEB1。
此外,生物信息学算法还显示,除了ZEB1还有其他基因,如TGF-β2、cyclin D2等。TGF-β2[20]和cyclin D2[21]在肿瘤的发生、发展过程中发挥了重要作用。 但是TGF-β2和cyclin D2在HNSCC中的作用还不清楚。我们计划在下一步的研究中,首先分析TGF-β2和cyclin D2 mRNA或蛋白在HNSCC的表达水平,并试图寻找TGF-β2和cyclin D2的表达水平与miR-141的表达水平是否具有相关性,然后通过双荧光报告基因的方法证实miR-141是否能靶向抑制TGF-β2和cyclin D2。
在本次研究中,我们仅证明了miR-141在HNSCC中能够抑制细胞增殖,miR-141是否还参与细胞凋亡及调控细胞周期仍需要进一步证明。
| [1] | Pignon J P, Bourhis J, Domenge C, Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer[J]. Lancet, 2000, 355: 949-955. |
| [2] | Suh Y, Amelio I, Guerrero Urbano T, Tavassoli M. Clinical update on cancer: molecular oncology of head and neck cancer[J]. Cell Death Dis, 2014, 5: e1018. |
| [3] | Kim V N, Han J, Siomi M C. Biogenesis of small RNAs in animals [J]. Nat Rev Mol Cell Biol, 2009, 10: 126-139. |
| [4] | Bartel D P. MicroRNAs: target recognition and regulatory functions [J]. Cell, 2009, 136: 215-233. |
| [5] | Valencia-Sanchez M A, Liu J, Hannon G J, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs[J]. Genes Dev, 2006, 20: 515-524. |
| [6] | Iorio M V, Croce C M. MicroRNAs in cancer: small molecules with a huge impact [J]. J Clin Oncol, 2009, 27: 5848-5856. |
| [7] | Yan B, Fu Q, Lai L, Tao X, Fei Y, Shen J, et al. Downregulation of microRNA 99a in oral squamous cell carcinomas contributes to the growth and survival of oral cancer cells [J]. Mol Med Rep, 2012, 6: 675-681. |
| [8] | Harris T, Jimenez L, Kawachi N, Fan J B, Chen J, Belbin T, et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas [J]. Am J Pathol, 2012, 180: 917-928. |
| [9] | Kinoshita T, Nohata N, Fuse M, Hanazawa T, Kikkawa N, Fujimura L, et al. Tumor suppressive microRNA-133a regulates novel targets: moesin contributes to cancer cell proliferation and invasion in head and neck squamous cell carcinoma [J]. Biochem Biophys Res Commun, 2012, 418: 378-383. |
| [10] | Zhu Z M, Xu Y F, Su Q J, Du J D, Tan X L, Tu Y L, et al. Prognostic significance of microRNA-141 expression and its tumor suppressor function in human pancreatic ductal adenocarcinoma [J]. Mol Cell Biochem, 2014, 388: 39-49. |
| [11] | Mateescu B, Batista L, Cardon M, Gruosso T, de Feraudy, Mariani O, et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response [J]. Nat Med, 2011, 17: 1627-1635. |
| [12] | Zhou X, Xia Y, Su J, Zhang G. Down-regulation of miR-141 induced by Helicobacter pylori promotes the invasion of gastric cancer by targeting STAT4[J]. Cell Physiol Biochem, 2014, 33:1003-1012. |
| [13] | Wu N, Liu C, Bai C, Han Y P, Cho W C, Li Q. Over-expression of deubiquitinating enzyme USP 14 in lung adenocarcinoma promotes proliferation through the accumulation of β-catenin [J]. Int J Mol Sci, 2013, 14: 10749-10760. |
| [14] | Wu N, Zhang C, Bai C, Han Y P, Cho W C, Li Q. MiR-4782-3p inhibited non-small cell lung cancer growth via USP14[J]. Cell Physiol Biochem, 2014, 33: 457-467. |
| [15] | Song B, Zhang C, Li G, Jin G, Liu C. MiR-940 inhibited pancreatic ductal adenocarcinoma growth by targeting MyD88[J]. Cell Physiol Biochem, 2015, 35: 1167-1177. |
| [16] | Zhao G, Wang B, Liu Y, Zhang J G, Deng S C, Qin Q, et al. MiRNA-141, downregulated in pancreatic cancer, inhibits cell proliferation and invasion by directly targeting MAP4K4 [J]. Mol Cancer Ther, 2013, 12: 2569-2580. |
| [17] | Gao Y C, Wu J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer [J]. Tumour Biol, 2015, 36: 4843-4850. |
| [18] | Zuo Q F, Zhang R, Li B S, Zhao Y L, Zhuang Y, Yu T, et al. MicroRNA-141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co-activator with PDZ-binding motif, TAZ[J]. Cell Death Dis, 2015, 6: e1623. |
| [19] | Wellner U, Schubert J, Burk U C, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs [J]. Nat Cell Biol, 2009, 11: 1487-1495. |
| [20] | Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina H G, et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RⅢ and p38α/β signalling [J]. Nat Cell Biol, 2013, 15: 1351-1361. |
| [21] | Koyama-Nasu R, Nasu-Nishimura Y, Todo T, Ino Y, Saito N, Aburatani H, et al. The critical role of cyclin D2 in cell cycle progression and tumorigenicity of glioblastoma stem cells [J]. Oncogene, 2013, 32: 3840-3845. |
 2015, Vol. 36
2015, Vol. 36


