2. 解放军309医院骨科, 北京 100091;
3. 解放军94826部队野战医院, 上海 200433;
4. 兰州军区总医院骨科, 兰州 730050;
5. 解放军71677部队卫生队, 安阳 450000
2. Department of Orthopedics, No. 309 Hospital of PLA, Beijing 100091, China;
3. Hospital of No. 94826 Troop of PLA, Shanghai 200433, China;
4. Department of Orthopedics, General Hospital, PLA Lanzhou Military Area Command, Lanzhou 730050, Gansu, China;
5. Medical Team, No. 71677 Troop of PLA, Anyang 450000, Henan, China
骨修复材料是进行骨移植、修复骨缺损的重要材料。自体骨虽然一直是骨修复材料的金标准[1],但存在供体不足、患者的二次损伤、出血量高、供骨部位容易出现并发症等缺点,而异体骨取代自体骨移植存在着排斥反应等风险,因此越来越多的研究关注于人工骨修复材料[2, 3, 4]。
天然骨组织主要是羟基磷灰石(hydroxyl apatite,HA)与胶原的复合体。HA具有良好生物相容性和生物活性,植入骨组织后能在界面上和骨形成很强的化学性键合,具有骨传导和骨诱导性,HA陶瓷已用于临床上骨的修复替换,但其脆性、不容易吸收的缺点制约了HA的应用范围[5, 6]。高分子材料如聚乳酸 (polylactic acid,PLA)、聚己内酯(poly-ε-caprolactone,PCL)、聚乙交酯(polyglycolide acid,PGA)等亦是目前骨移植材料的研究热点之一,这些高分子材料被证明具有良好的生物相容性与生物降解性,但不足之处在于生物活性弱。相比于单纯的HA或高分子材料,复合型材料兼具有两种材料优点的同时又能相互弥补两者的不足,是目前骨移植材料的研究热点之一[7, 8]。
近年来,快速成形 (rapid prototyping,RP)技术作为新型材料加工技术迅猛发展,它借助于医学成像系统(CT、MRI)、计算机设计软件(CAD)、数字转换系统,能迅速而准确地产生复杂的三维物体模型[9]。选择性激光烧结(selective laser sintering,SLS)技术是RP技术的一种,它可以通过制备参数的设计对多孔支架的外形及内部结构如孔隙率、孔尺寸、孔分布等影响多孔支架骨修复效果的关键特征实现精确和灵活的控制,具有材料制备时间短、可批量化生产、能满足临床上患者不同的骨缺损修复需求从而实现个体适配性等优点[10, 11]。本研究旨在运用SLS构建不同成分配比纳米羟基磷灰石(Nano-hydroxyapatite,Nano-HA)/PCL人工骨支架,并通过一系列生物力学及细胞学实验[12, 13],对其力学性能、生物相容性及生物活性进行探索与研究。
1 材料和方法 1.1 主要材料与设备 HA购自Emperor公司(中国南京),纯度大于99.5%,扫描电子显微镜(scanning electron microscope,SEM)下呈长棱形,长(150±20) nm,宽(20±5) nm。PCL购自Esun公司(中国深圳),乳白色颗粒,相对分子质量50 000,直径3~5 mm,熔点58~60℃,比重1.08~1.12 g/cm3,纯度大于99%。两种材料均为医用级别。V型混合器(VH-5)购自上海高桥试验机厂有限公司,SLS机器(AFS-500)购自北京隆源自动成型系统有限公司。
1.2 制备Nano-HA/PCL复合材料通过深温冷冻法将PCL球状颗粒研磨成微米级别的粉末,粒径范围500~800 μm[10]。再使用V型混合器将Nano-HA与PCL按不同质量比(10:90,15:85,20:80)分别进行混合,30 r/min混合1 h,保证两种原材料粉末混合均匀,SEM观察混合效果。
1.3 Nano-HA/PCL人工骨支架的制备及表征观察应用CAD软件来设计人工骨支架的模型:外观结构为直径6 mm、高15 mm的圆柱形,内部结构则为规整分布且相互连通的孔结构,孔径为800 μm。保存支架模型文件。将混合均匀的Nano-HA/PCL粉末倒入SLS机器的原料箱中。结合Wiria等[14]的研究及预实验结果,将SLS的参数设定如下:激光束直径150 μm,内部扫描线功率4.5 W、边框扫描线功率3 W、支撑扫描线功率3 W,内部扫描速度1.25 m/s、边框扫描速度0.55 m/s、支撑扫描速度1.33 m/s,按层厚为150 μm来进行加工。烧结完成后,样品置于加工舱中冷却1 h方可取出,吹风法去除样品孔隙结构中残留的原材料粉末。同法制备PCL人工骨支架。
1.4 孔隙率及力学性能的检测根据阿基米德原理[15]计算支架的平均孔隙率,每组测量5个。计算公式为:孔隙率=(m2-m1)/(m2-m3)×100%。其中m1为支架在空气中的干质量,m2为支架在空气中的饱和湿质量,m3为支架悬浮在液体中的湿质量。采用电子万能材料试验机对支架样品的力学强度进行测定,读取支架被压缩至原长度1/2时的抗压强度,每组测量5个样品。
1.5 兔骨髓间充质干细胞(MSCs)的分离与培养4周龄新西兰兔过量麻醉处死,取其双侧股骨,彻底去除周围附着的肌肉组织,小勺刮取骨髓组织,使用0.2%胰蛋白酶液消化1 h后终止,PBS液清洗3次,然后使用0.2 g/L Ⅱ型胶原酶和0.04 g/L Ⅱ型DNA酶37℃消化4 h,过滤、清洗,后以含体积分数为10%胎牛血清的DMEM/F12培养液将细胞密度调整到1×108/mL,接种于培养瓶内,置于37℃、5% CO2孵育箱中培养。7 d后首次换液,以后每周换液2次。细胞传至第3代时即可用于实验。
1.6 实验分组及细胞接种实验分为HA 质量分数分别为5%、10%、15%的 Nano-HA/PCL支架+MSCs共培养组(5% Nano-HA/PCL组、10% Nano-HA/PCL组、15% Nano-HA/PCL组),PCL支架+MSCs共培养组(PCL组)和MSCs单独培养组(空白对照组)。将各组人工骨支架切割切成5 mm薄片后置入培养瓶中,环氧乙烷熏蒸灭菌防止污染。将传至第3代的兔MSCs用胰蛋白酶消化脱壁,制成密度为1×105 /mL的细胞悬液,Nano-HA/PCL组与PCL组均将MSCs接种于24孔板中的支架上,空白对照组直接将MSCs接种于24孔细胞培养板中。各组所使用的培养液均为成骨诱导培养液(DMEM/F12+10%体积分数的胎牛血清+0.1 μmol/L地塞米松+10 mmol/L β-甘油磷酸+50 μmol/L抗坏血酸)。细胞接种后,将培养瓶置于37℃、5% CO2的细胞培养箱中培养。12 h后取出支架置入2.5%的戊二醛溶液中浸泡24 h,而后乙醇梯度脱水并自然干燥,SEM下观察各组人工骨支架上的细胞黏附情况。
1.7 检测指标 1.7.1 细胞毒性和增殖能力使用MTT比色法测定人工骨支架对细胞的毒性作用以及细胞在支架上的增殖能力。各组分别于接种后第1、4、7天取5孔,弃原培养液,每孔加入MTT溶液80 μL,再加入无血清的DMEM/F12培养液400 μL,37℃、5% CO2细胞培养箱中孵育4 h后,弃上清,每孔加入二甲亚砜600 μL,振荡15 min,各孔吸取150 μL溶液至96孔细胞培养板中,酶标仪检测D值,测定波长为490 nm。
1.7.2 碱性磷酸酶(ALP)的表达各组分别于接种后第1、7、14天取5孔,弃原培养液,PBS液冲洗2次,加入0.25% 胰蛋白酶消化3 min,加入无血清的DMEM/F12培养液终止消化,吸入离心管中,1 328×g离心5 min,弃上清,每管加入200 μL 2%的TritionX-100,置于4℃冰箱过夜以裂解细胞。第2天反复吹打后,再次以1 328×g离心10 min,而后取50 μL上清液按照ALP试剂盒(AP0100,Sigma)说明操作。酶标仪检测D值,测定波长为520 nm。
1.7.3 茜素红法钙结节染色各组分别于接种后第7、14、21天取3孔,弃原培养液,每孔中加入4%多聚甲醛溶液,覆盖孔板底部即可。固定1 h后移除固定液,PBS液清洗3次,而后加入茜素红液(1%浓度,pH 3.7),覆盖孔板底部即可,37℃ 恒温振荡箱中振荡45 min,移除茜素红液,PBS再清洗3次以彻底移除茜素红液,倒置显微镜下观察染色结果。
1.8 统计学处理采用SPSS 17.0统计软件进行统计学分析,所有数据均以 x±s表示。多组均数的多重比较用方差分析(ANOVA)的LSD检验或Dunnett检验。检验水准(α)为0.05。
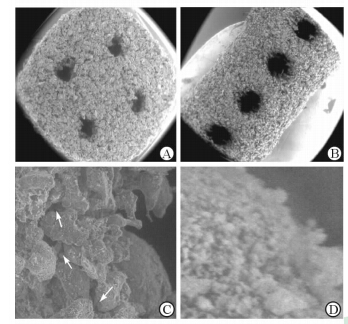
2 结 果 2.1 表面形貌和微观结构分析结果显示,支架的模型被设计成圆柱状,支架内含有相互联通的孔隙结构(图1A);烧结完成后,支架高15 mm,底面直径为6 mm,在支架的侧面与底面均可以观察到预先设计好的孔隙结构,同时支架的孔隙中无粉末残留(图1B、1C)。SEM下观察,相互粘结的PCL颗粒构成该人工骨支架的骨架,同时在支架的底面及侧面可以清楚观察到预先所设计好的较大的孔隙结构,孔隙直径约500~800 μm(图2A、2B)。此外,相互粘连的PCL颗粒之间还存在着微小的、相互连通的不规则孔隙结构(图2C);同时在PCL颗粒表面,还非常均匀地黏附着Nano-HA颗粒粉末(图2D)。

|
图 1 Nano-HA/PCL人工骨支架的模型图 (A)、实物的侧面(B)与底面(C) Fig 1 Computer-aided design models of the scaffolds(A),lateral view (B) and bottom view (C) of sintered scaffolds Nano-HA: Nano-hydroxyapatite; PCL: Poly-ε-caprolactonee |
|
图 2 Nano-HA/PCL人工骨支架的扫描电镜图 Fig 2 Scanning electronic microscopy micrographs of the prepared scaffolds A,B: Bottom view (A) and lateral view (B) of the nano-hydroxyapatite/poly-ε-caprolactone (PCL) scaffolds with well-ordered and interconnected macropores from 600 μm to 800 μm; C: Microstructure of the scaffolds. The micropores (white arrows) appeared between PCL particles with pore sizes of several microns in diameter; D: The PCL particles were evenly attached to the nanoscale hydroxyapatite particles. Nano-HA: Nano-hydroxyapatite; PCL: Poly-ε-caprolactone. Original magnification: ×50 (A,B),×1 000 (C),×5 000 (D) |
结果显示,Nano-HA/PCL人工骨支架较PCL人工骨支架孔隙率有所下降( P<0.05),力学强度也得到了明显改善(P<0.05)。 随着HA含量比例增加,Nano-HA/PCL人工骨支架的孔隙率逐渐降低( P<0.05),但仍然处于比较理想的范围;而力学强度却明显改善(P<0.05),已经达到了松质骨的强度范 围。详见表1。
|
|
表 1 各组人工骨支架的孔隙率和抗压强度 Tab 1 Porosity and compressive strength of the scaffolds in each group |
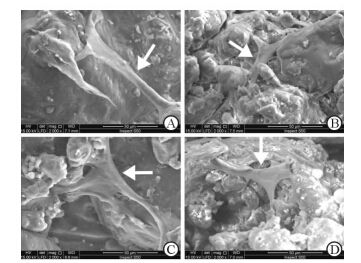
细胞接种后12 h,Nano-HA/PCL组及PCL组均可以观察到细胞很好地黏附于支架表面(图3),同时所有的细胞均表现出良好的延展,表明Nano-HA/PCL及PCL人工骨支架均具有优良的生物相容性,适于后续的体外细胞实验。
|
图 3 各组人工骨支架表面细胞的黏附情况 Fig 3 Cell adhesion of scaffords in each group under scanning electron microscopy A: PCL group; B: 5% Nano-HA/PCL group; C: 10% Nano-HA/PCL group; D: 15% Nano-HA/PCL group. Nano-HA: Nano-hydroxyapatite; PCL: Poly-ε-caprolactone. The white arrows are the human bone marrow stromal cells |
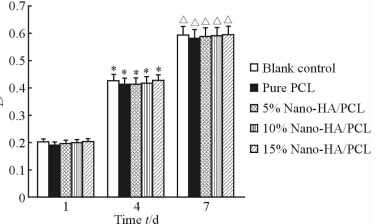
结果(图4)显示,接种后第1、4、7天时各组的D值均逐渐提高;但在每个时间节点,各组之间的D值差异无统计学意义( P >0.05),表明Nano-HA/PCL人工骨支架具有良好的生物相容性,能为细胞生长提供合适的环境。
|
图 4 MTT法测得各组不同时间点的D值 Fig 4 Density of MTT test for each group at days 1,4 and 7 of culture Nano-HA: Nano-hydroxyapatite; PCL:Poly-ε-caprolactone. *P<0.05 vs the same group at day 1; △ P <0.05 vs the same group at day 4. n=5, x±s |
结果(图5)表明,接种后1 d各组的ALP表达均较低,差异无统计学意义(P>0.05),但随着培养时间的延长,Nano-HA/PCL组ALP的表达水平逐渐高于PCL组与空白对照组( P <0.05),且含Nano-HA的比例越高,ALP的表达也越强( P <0.05),表明Nano-HA具有良好的生物活性,可以促进兔MSCs向成骨细胞方向分化。

|
图 5 各组不同时间点ALP的表达 Fig 5 Alkaline phosphatase in each group at days 1,7,and 14 Nano-HA: Nano-hydroxyapatite; PCL:Poly-ε-caprolactone; ALP: alkaline phosphatase. *P < 0.05 vs blank control group at same time; △ Plt 0.05 vs PCL group at same time; ▲ P < 0.05 vs 5% Nano-HA/PCL group at same time; ▽ P < 0.05 vs 10% Nano-HA group at same time. n=5,x±s |
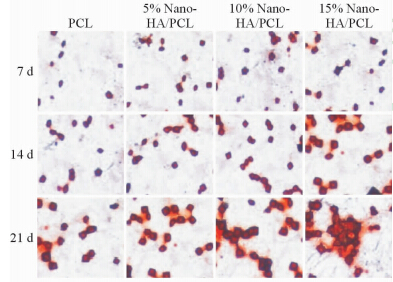
茜素红染色结果(图6)显示,随着时间的推移,各组植入区域组织中的钙结节数量均增多。同一时间点Nano-HA/PCL组钙结节的数目多于PCL组,且随着Nano-HA含量的增加,钙结节的数量增多,表明Nano-HA可以促进MSCs的成骨分化。
|
图 6 各组不同时间点钙结节茜素红法染色图 Fig 6 Alizarin Red S staining of calcium nodules in each group at different time points Nano-HA: Nano-hydroxyapatite; PCL: Poly-ε-caprolactone. Original magnification: ×20 |
三维多孔支架内部优良的3D结构为细胞的生长和组织的重建提供了一个理想的空间。已有研究表明,当支架的孔隙率大于70%且支架的孔径在100~900 μm之间时,有利于负载于其上的细胞增殖与分化(特别是成骨分化)[16, 17]。为了更好地模拟天然骨的形态结构,本研究使用了纳米级的HA来作为人工骨中无机成分的原材料,以期能提高人工骨支架的力学性能与生物活性[18, 19]。本次研究成功地利用SLS技术构建出了Nano-HA/PCL人工骨支架,该支架孔隙率为70%~75%,抗压强度为5.5 596~6.9 764 MPa,虽然未能达到皮质骨的强度(80~150 MPa),但是已经处于松质骨的强度范围[20],这可能是由材料高度的孔隙率及PCL颗粒本身较差的力学强度导致的。而Nano-HA/PCL人工骨支架的力学强度强于PCL人工骨支架,表明无机与有机材料的结合可以增加支架的力学强度。
SLS技术作为3D打印技术的一种,将影像学与计算机技术相结合,可以根据骨缺损的形状与生物力学的要求制作出高度个体适配性的人工骨支架。本研究制备的Nano-HA/PCL人工骨支架除了具有预先设计的、相互连通的孔隙结构外还存在着许多不规则但也相互连通的微孔隙结构。这些四通八达的孔隙结构为血管的生长、营养物质的输送以及细胞代谢废物的排出提供了十分有利的条件[21, 22]。通过细胞黏附、细胞毒性、细胞增殖的实验结果可以看出,各组支架均展现出了非常优秀的生物相容性,细胞毒性极小。而通过ALP表达以及茜素红染色的实验结果可以看出,Nano-HA/PCL人工骨支架相比于PCL人工骨支架具有更加良好的生物活性,能很好地促进MSCs的成骨分化,且随着Nano-HA含量比例的增加,Nano-HA/PCL人工骨支架生物活性也愈强[7]。
本研究成功利用SLS技术制备出了Nano-HA/PCL人工骨支架,并通过一系列力学与细胞学实验证明了该支架具有较好的力学强度、生物相容性与生物活性,为后续动物体内实验研究奠定了一定的前期基础。
| [1] | Williams D. Benetit and risk in tissue engineering[J]. Mater Today, 2004, 7: 24-29. |
| [2] | Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory's experience [J]. Acc Chem Res, 2000, 33: 94-101. |
| [3] | Shin H, Jo S, Mikos A G. Biomimetic materials for tissue engineering [J]. Biomaterials, 2003, 24: 4353-4364. |
| [4] | Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas A M, de Ruiter A, et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting [J]. Proc Natl Acad Sci U S A, 2010, 107: 13614-13619. |
| [5] | Ma P X, Zhang R, Xiao G, Franceschi R. Engineering new bonetissue in vitro on highly porous poly(alpha-hydroxyl acids)/hydroxyapatite composite scaffolds[J]. J Biomed Mater Res, 2001, 54: 284-293. |
| [6] | Ishaug S L, Crane G M, Miller M J, Yasko A W, Yaszemski M J, Mikos A G, et al. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds[J]. J Biomed Mater Res, 1997, 36:17-28. |
| [7] | Causa F, Netti P A, Ambrosio L, Ciapetti G, Baldini N, Pagani S, et al. Poly-epsilon-caprolactone/hydroxyapatite composites for bone regeneration: in vitro characterization and human osteoblast response[J]. J Biomed Mater Res A, 2006, 76: 151-162. |
| [8] | Wutticharoenmongkol P, Sanchavanakit N, Pavasant P, Supaphol P. Preparation and characterization of novel bone scaffolds based on electrospun polycaprolactone fibers filled with nanoparticles[J]. Macromol Biosci, 2006, 6: 70-77. |
| [9] | Hollister S J. Porous scaffold design for tissue engineering[J]. Nat Mater, 2005, 4: 518-524. |
| [10] | Williams J M, Adewunmi A, Schek R M, Flanagan C L, Krebsbach P H, Feinberg S E, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering[J]. Biomaterials, 2005, 26: 4817-4827. |
| [11] | Tan K H, Chua C K, Leong K F, Cheah C M, Gui W S, Tan W S, et al. Selective laser sintering of biocompatible polymers for applications in tissue engineering[J]. Biomed Mater Eng, 2005, 15(1-2): 113-124. |
| [12] | Baker S C, Rohman G, Southgate J, Cameron N R. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering[J]. Biomaterials, 2009, 30: 1321-1328. |
| [13] | Suárez-González D, Barnhart K, Migneco F, Flanagan C, Hollister S J, Murphy W L.Controllable mineral coatings on PCL scaffolds as carriers for growth factor release[J]. Biomaterials, 2012, 33: 713-721. |
| [14] | Wiria F E, Leong K F, Chua C K, Liu Y. Poly-e-caprolactone/hydroxyapatite for tissue engineering scaffold fabrication via selective laser sintering[J]. Acta Biomaterialia, 2007, 3: 1-12. |
| [15] | Xu C, Su P, Chen X, Meng Y, Yu W, Xiang A P, et al. Biocompatibility and osteogenesis of biomimetic bioglass-collagen-phosphatidylserine composite scaffolds for bone tissue engineering[J]. Biomaterials, 2011, 32: 1051-1058. |
| [16] | Del Real R P, Wolke J G, Vallet-Regi M, Jansen J A. A new method to produce macropores in calcium phosphate cements[J]. Biomaterials, 2002, 23: 3673-3680. |
| [17] | Ma P X. Scaffolds for tissue fabrication[J]. Mater Today, 2004, 7: 30-40. |
| [18] | Poinern G E, Brundavanam R K, Mondinos N, Jiang Z T. Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method[J]. Ultrason Sonochem, 2009, 16: 469-474. |
| [19] | Hellmich C, Ulm F J. Average hydroxyapatite concentration is uniform in the extracollagenous ultrastructure of mineralized tissues: evidence at the 1-10-microm scale[J]. Biomech Model Mechanobiol, 2003, 2: 21-36. |
| [20] | Anseth K S, Shastri V R, Langer R. Photopolymerizable degradable polyanhydrides with osteocompatibility[J]. Nat Biotechnol, 1999, 17: 156-159. |
| [21] | Wang Y W, Wu Q, Chen G Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds[J]. Biomaterials, 2004, 25: 669-675. |
| [22] | Xin X, Hussain M, Mao J J. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold[J]. Biomaterials, 2007, 28: 316-325. |
 2015, Vol. 36
2015, Vol. 36


