2. 甘肃中医学院附属医院耳鼻咽喉头颈外科, 兰州 730020
2. Department of Otolaryngology-Head and Neck Surgery, Affiliated Hospital of Gansu University of TCM, Lanzhou 730020, Gansu, China
变应性鼻炎的患病率逐年升高,根据2010 ARIR指南[1],全球有超过5亿变应性鼻炎患者。纤毛的结构与功能是决定鼻黏膜基本生理功能的重要因素,目前对变应性鼻炎鼻黏膜超微结构改变已有较多的认识,但对变应性鼻炎纤毛超微结构的长周期变化仍有待深入观察。甘草次酸(glycyrrhetinic acid,GA)是传统中药甘草的有效成分甘草酸在体内的水解产物,现代药理学研究发现甘草次酸具有抗炎、抗过敏、抗病毒、抗溃疡、抗氧化、抗心律失常、镇咳、降血脂、改善胰岛素抵抗以及免疫调节等多种药理作用,其抗变态反应作用亦得到一定认识[2, 3],但它对变应性鼻炎的治疗作用及对鼻黏膜纤毛超微结构的长期影响尚未见报道。本研究建立变应性鼻炎大鼠模型并给予甘草次酸干预,观察鼻黏膜纤毛超微结构的进行性损害改变及甘草次酸对变应性鼻炎大鼠鼻黏膜重塑的影响。 1 材料和方法 1.1 主要试剂和仪器
卵清蛋白(ovalbumin,OVA,A5503,5 g/瓶,Ⅴ级,美国Sigma公司,批号1001445093);氢氧化铝[Al(OH)3,500 g/瓶,AR级,天津市致远化学试剂有限公司];氯雷他定(10 mg/片,四川奥邦药业有限公司,生产批号121109);18β-甘草次酸(18β-glycyrrhetinic acid,18β-GA,500 g/袋,AR级,甘肃泛植生物科技有限公司,生产批号12081603)。扫描电镜(JEM-6380型)、透射电镜 (JEM-1230型)均为日本电子株式会社产品。 1.2 实验动物
96只SPF级健康Wistar大鼠,雌雄各半,4~6周龄,体质量180~220 g(甘肃中医学院SPF动物实验室提供),随机分为空白组、模型组、氯雷他定组和18β-GA,每组24只,饲养于甘肃中医学院SPF动物中心。 1.3 模型建立
造模方法参照文献[4],将0.3 mg OVA和30 mg Al(OH)3加生理盐水配成1 mL混悬液,对模型组、氯雷他定组和18β-GA组大鼠行腹腔注射基础致敏,空白组以等量生理盐水替代,隔日1次×7次,共14 d;自第15天开始进行鼻腔激发,方法如下:用微量进样器以2% OVA滴鼻,50 μL/侧×2侧鼻腔,空白组以等量生理盐水替代,每日1次×7 d。末次鼻腔激发后观察大鼠喷嚏、流涕和挠鼻表现,计数30 min内喷嚏及挠鼻次数,根据动物行为学评分标准进行评分,判断造模是否成功。(1)鼻痒:轻度(轻擦鼻数次)为1分,重度(抓挠鼻、面不止,动作快而反复)为2分;(2)喷嚏:1~3个为1分,4~10个为2分,11个以上为3分;(3)清涕:流至前鼻孔为1分,超过前鼻孔为2分,流涕满面为3分。叠加量化评分超过5分者视为造模成功。 1.4 给药干预
实验第22天开始给药干预。以临床用药氯雷他定胶囊(10 mg/片,1片/次,1次/d)及复方甘草酸苷片用药量(每片含甘草酸25 mg,3片/次,3次/日,每日甘草酸总用量225 mg)为参考,根据动物体表面积转换计算公式确定给药剂量:氯雷他定组每只大鼠给予氯雷他定1 mg/kg溶于2 mL生理盐水灌胃,18β-GA组给予18β-GA 25 mg/kg混悬于2 mL生理盐水灌胃(因18β-GA不溶于水,先按体质量计算18β-GA给药量,将小玻璃瓶清洗消毒并干燥,在分析天平上归零,将粉末状18β-GA缓慢加入其中,精确称量,然后加入2 mL生理盐水,在振荡混匀器上充分混匀),空白组和模型组以等量生理盐水替代,1次/d,共10周。在此10周时间内,模型组、氯雷他定组和18β-GA组以2% OVA滴鼻维持激发,50 μL/侧,2次/周,空白组以等量生理盐水替代。 1.5 观察指标
给药第2、4、6和10周后每组随机取6只大鼠,在末次滴鼻后对各组行为学叠加量化评分。末次给药24 h后将大鼠以10%水合氯醛(3 mL/kg)腹腔注射麻醉,剪毛、打开胸腔,心脏放血后立即以4%多聚甲醛50 mL快速心脏灌注,然后断头,迅速打开鼻背取双侧鼻腔黏膜,用0.1 mol/L PBS漂洗后放入2.5%戊二醛溶液、4℃固定24 h制备扫描及透射电镜标本。透射电镜标本经2.5%戊二醛固定、1%锇酸固定、丙酮脱水和浸透包埋,光镜下定位后超薄切片,用JEM-1230型透射电镜观察。扫描电镜标本经2.5%戊二醛固定、1%锇酸固定、冷冻干燥、粘样及喷金镀膜后采用JEM-1230型扫描电镜观察。 1.6 统计学处理
采用SPSS17.0统计软件分析数据,计量资料以 x±s表示,行单因素方差分析,用最小显著差异法(LSD法)比较两两差异。检验水准(α)为0.05。
2 结 果 2.1 动物模型评估
每只造模大鼠均出现反复抓鼻、喷嚏和流涕症状,叠加评分均超过5分,根据动物造模标准,认为造模成功。空白组大鼠偶有喷嚏及轻微抓鼻,未出现明显症状和鼻部体征。 2.2 各组大鼠鼻部症状比较
给药过程中,18β-GA组和氯雷他定组大鼠喷嚏次数、抓鼻次数和鼻分泌物逐渐减少,干预2周后18β-GA组和氯雷他定组的抓鼻、喷嚏及鼻分泌物评分均低于模型组,差异有统计学意义(P<0.05);18β-GA组和氯雷他定组的挠鼻症状评分均高于空白组(P<0.05),而喷嚏数和鼻分泌物与空白组相近(P>0.05);两个干预组(18β-GA组和氯雷他定组)末次抓鼻、喷嚏数及鼻分泌物计分均接近,两组间差异无统计学意义(P>0.05 )。至干预4周以后,18β-GA组和氯雷他定组大鼠挠鼻、喷嚏和鼻分泌物评分与空白组差异均无统计学意义(P>0.05);且这两个干预组之间差异亦无统计学意义(P>0.05)。见表 1。
|
|
表 1 给药后各时间点各组大鼠鼻部症状评分比较 Tab 1 Comparison of symptom scores in each group after administration of agents at different time points |
空白组鼻黏膜纤毛密集、整齐、摆动规律,纤毛直径及长度一致,“9×2+2”微管结构规则(图 1A、1E)。
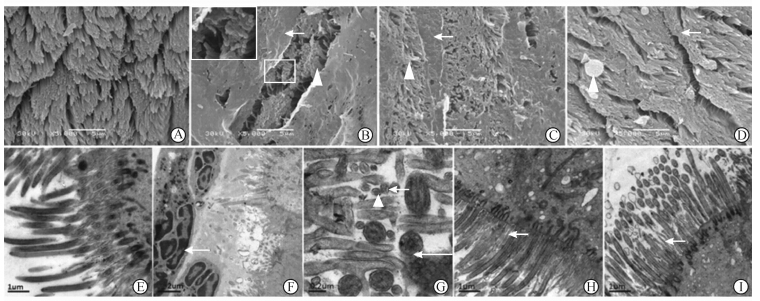 | 图 1 干预2周后各组纤毛超微结构比较 Fig 1 Comparison of cilia ultrastructure between different groups 2 weeks after administration of corresponding agents A:Normal control group; B:Model group,thick mucus blanket on the top of cilia in large areas(→),cilia matting together (△); C:Loratadine treatment group,thick mucus blanket on the top of some cilia (→),a few cilia matting together(△); D:18β-glycyrrhetinic acid treatment group,dense cilia with a few matting together (→),goblet cell (△) ; E:Normal control group; F:Model group,thick mucus blanket containing neutrophils (→) and other inflammatory cells; G: Model group,showing decreased microtubules in normal-sized cilia(long arrow) and small cilia (△),rupture of ciliary membrane (short arrow); H:Loratadine group,dense and normal cilia with a few short and abnormal cilia (→); I:18β-glycyrrhetinic acid treatment group,dense and normal cilia with a few short and abnormal cilia (→). Original magnification: ×5 000 (A,B,C,D),×10 000 (F),×20 000 (E,H,I),×80 000(G) |
维持激发2周后,模型组大鼠鼻黏膜黏液毯显著增厚、呈大片状广泛黏着覆盖于纤毛顶端,厚3~5 μm,呈中等电子密度,其中含中性粒细胞、单核细胞等炎性细胞(图 1B、1F);局部尚未被遮盖的纤毛较密集,但方向紊乱,纤毛顶端粘连成簇(图 1B),部分纤毛内残存数个微管、散乱分布或发生融合而计数不清,中央微管融合或缺失,偶见纤毛膜破裂,大量正常直径(0.2~0.5 μm)的纤毛中掺杂有直径0.08~0.12 μm(根据标尺计算)的短小纤毛,后者仅有1组中央微管,或无微管而呈空心圆状(图 1G)。氯雷他定组鼻黏膜纤毛顶端亦可见细条带状黏胶层增厚覆盖局部纤毛,但覆盖面积明显小于模型组;未被覆盖的纤毛密集,局部纤毛散乱或粘连呈细束,基体完整,纤毛膜完整,微管结构清晰,在大量直径>0.2 μm的纤毛中偶见直径0.08~0.12 μm的短小纤毛(图 1C、1H)。18β-GA组与氯雷他定组电镜下表现相似,但纤毛顶端未见条带状增厚的黏液毯覆盖(图 1D、1I)。
维持激发4周后,模型组在2周时广泛黏附于纤毛顶端增厚的黏液毯于4周时消失,纤毛变短、纤细、弯曲倒伏,较2周时更加散乱,且粘连呈簇,局部纤毛脱落致使纤毛上皮细胞及杯状细胞裸露(图 2A);纤毛根部基体缺失,细胞膜局部突起并伸出数个长短不一、方向杂乱的纤毛(图 2D),视野内未见直径0.2 μm以上的纤毛,而直径0.08~0.12 μm的短小纤毛显著增多,其内微管无 “9×2+2”轮状结构、散乱分布于纤毛局部或融合聚集成团而难以计数,部分纤毛缺失微管结构,纤毛膜破裂(图 2E)。氯雷他定组纤毛比2周时略密集,2周时覆盖于纤毛顶端的条带状黏胶层在4周时已消失(图 2B),纤毛排列整齐,局部有束状粘连,微管结构清晰,形态一致的纤毛之间仍散在少量短小纤毛,数量较2周时减少(图 2F)。18β-GA组密集整齐的纤毛中局部少量纤毛方向散乱,2周时较多见的局部纤毛束状粘连在4周时明显减少(图 2C),纤毛微管结构清晰,偶见短小纤毛(图 2G)。
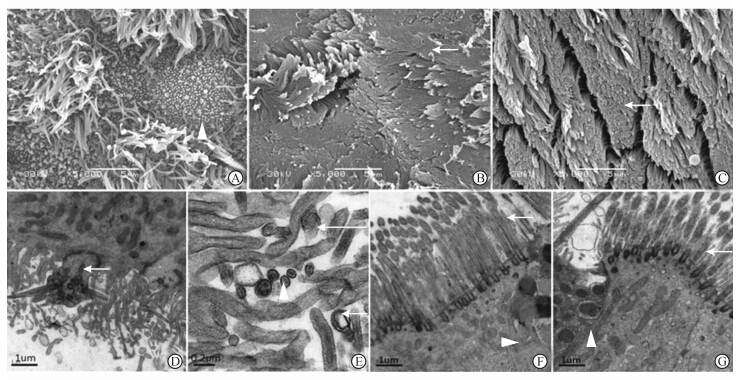 | 图 2 干预4周后各组纤毛超微结构比较 Fig 2 Comparison of cilia ultrastructure between different groups 4 weeks after administration of corresponding agents A:Model group,showing loss of cilia with remaining cilia matting together and exposure of goblet cells (△); B:Loratadine treatment group,dense cilia with a few matted together (→); C:18β-glycyrrhetinic acid treatment group,dense cilia with a few matted together (→); D:Model group,showing short and abnormal cilia,abnormal protrusion of membrane and basal body without cilium (→); E: Model group,rupture of cilia membrane (long arrow),cilia without microtubule (short arrow) and small cilia with only one pair of microtubules in the center (△); F:Loratadine treatment group,dense and normal cilia with a few short and abnormal cilia (→),goblet cell (△); G: 18β-glycyrrhetinic acid treatment group,dense and normal cilia arranged with a few short and abnormal cilia (→),goblet cell (△). Original magnification: ×5 000 (A,B,C),×20 000 (D,F,G),×80 000 (E) |
维持激发6周后,模型组鼻黏膜纤毛进一步脱落,部分纤毛细胞及杯状细胞裸露,残余纤毛弯曲倒伏、缠绕,局部仍残余簇状密集整齐的纤毛(图 3A),上皮细胞破损、降解,纤毛显著稀疏,于4周时数量较多的短小纤毛在6周时亦减少,残余纤毛融合,纤毛膜破裂较4周增多,纤毛内微管减少较4周时更进一步,视野内多见无微管结构且纤毛膜破损的缺损圆环状横切面(图 3D、3E)。氯雷他定组纤毛密集,偶见纤毛散乱,纤毛束状粘连较4周时减少,微管结构清晰(图 3B、3F)。18β-甘草次酸组纤毛较4周时略密集,排列较整齐,纤毛之间舒展、偶可见数个纤毛顶端粘连但未成束(图 3C),纤毛根部基体完整,纤毛膜完整,微管清晰(图 3G)。
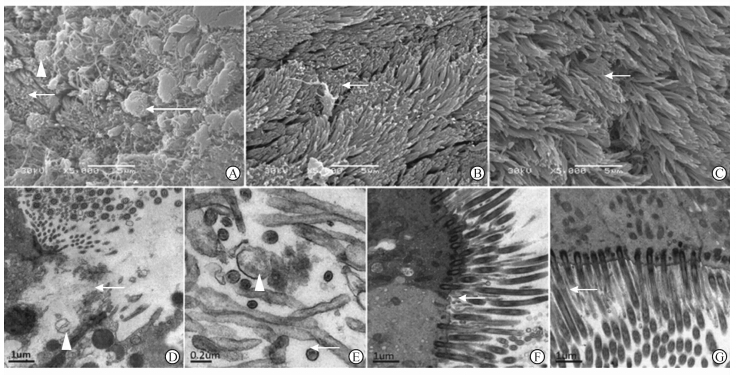 | 图 3 干预6周后各组纤毛超微结构比较 Fig 3 Comparison of cilia ultrastructure between different groups 6 weeks after administration of corresponding agents A:Model group,ciliary destruction as loss,lodging and matting together,with some cilia remaining normal (short arrow) and the exposure of goblet cell (long arrow) and ciliated cell (△); B:Loratadine treatment group,dense cilia with few matted together (→); C:18β-glycyrrhetinic acid treatment group,dense cilia with few matted together (→); D:Model group,extensive loss of cilia,cytolysis (→) and organelle degradation (△); E: Model group,rupture of ciliary membrane (△),short and abnormal cilia with only one pair of microtubules (→); F:Loratadine treatment group,dense and normal cilia with few short and abnormal cilia (→) ; G:18β-glycyrrhetinic acid treatment group,dense and normal cilia with few short and abnormal cilia (→). Original magnification: ×5 000 (A,B,C),×20 000 (D,F,G),×80 000 (E) |
维持激发10周后,模型组鼻黏膜上皮细胞纤毛脱落较6周时更显著,视野内呈大范围的纤毛稀疏,大量杯状细胞及纤毛上皮细胞裸露,残余的少量纤毛弯曲倒伏、粘连缠结呈网状(图 4A),上皮细胞破裂呈降解样改变、细胞内大量脂褐素堆积(图 4D)。残余纤毛大小不一、纤毛融合、纤毛膜破裂,稀疏的短小纤毛内仅残余中央二联微管或完全缺失后残留由纤毛膜形成的空心圆状(图 4E)。氯雷他定组纤毛密集,局部略杂乱(图 4B),微管结构清晰(图 4F)。18β-甘草次酸组纤毛密集、较整齐,偶见局部少量纤毛略杂乱,纤毛之间无粘连(图 4C),微管结构清晰(图 4G)。18β-甘草次酸组与氯雷他定组之间未见显著差别。
 | 图 4 干预10周后各组纤毛超微结构比较 Fig 4 Comparison of cilia ultrastructure between different groups 10 weeks after administration of corresponding agents A:Model group,showing extensive destruction of cilia,and the exposure of goblet cell (→)and ciliated cell (△); B:Loratadine treatment group,dense cilia with very few matted together; C:18β-glycyrrhetinic acid treatment group,dense cilia with very few matted together; D:Model group,rupture and degradation of ciliated cell (→),loss of cilia,absence of basal body (△); E: Model group,fusion of cilia and rupture of ciliary membrane (long arrow),small cilium with only one pair of microtubule (△),and absence of microtubule in cilia (short arrow); F:Loratadine treatment group:dense cilia; G:18β-glycyrrhetinic acid treatment group,dense cilia in one direction. Original magnification: ×5 000 (A,B,C),×20 000 (D,F,G),×80 000 (E) |
Wistar大鼠是建立AR模型的经典模式动物之一,Guibas等[5]、Ogawa等[6]均采用大鼠建立AR模型以研究药物对实验性变应性鼻炎及上气道变应性炎症的干预作用。韩菲等[7]、刘建国等[8]观察到变应性鼻炎大鼠鼻黏膜纤毛出现粘连、脱落、微管结构破坏等改变。 本研究的特点是观察长时间变应原激发下鼻黏膜纤毛超微结构的进行性改变以及GA对其变化的影响。实验预计设定为1~12周,期望观察到鼻黏膜纤毛超微结构从急性炎症期(1周)到慢性持续性炎症(长达3个月)的进展变化。但预实验发现干预1周时18β-GA组鼻黏膜超微结构病理损害未见显著改善,而从2周开始显现疗效,因此正式实验设计从干预2周开始取材;而在第10周时,模型组鼻黏膜超微结构已出现严重病理改变:纤毛大量脱落致使部分细胞表面几乎无纤毛残余、且纤毛上皮细胞破裂及细胞器降解,而18β-GA组鼻黏膜病理损害逐渐改善、至第10周时已与空白对照组无明显差异,故以10周为结束时间点。本研究发现,在变应原维持激发下,模型组鼻黏膜纤毛超微结构随着时间延长而出现一系列进展性病理改变:2周时以纤毛顶端增厚并包裹黏附中性粒细胞、单核细胞等大量炎性细胞的黏液毯为特征性改变,纤毛簇状粘连并出现短小纤毛;4周时部分纤毛脱落、细胞裸露,直径<0.2 μm的小纤毛增多而直径>0.2 μm的正常形态的纤毛则较2周时减少,于2周时偶见的微管减少、融合及纤毛膜破裂等表现在4周时明显增多;6周时纤毛进一步脱落,并出现细胞膜破裂,于4周时多见的短小纤毛在6周时亦明显减少;至10周时纤毛几乎完全脱落、细胞破裂溶解、细胞内大量脂褐素堆积,残余的纤毛几乎均为直径0.08~0.12 μm的短小纤毛,其数量亦较6周时减少,其内仅含1组中央微管或无微管而呈空管状,偶见残存>0.2 μm者,其内微管亦较少或发生融合而计数不清。
鼻黏膜纤毛是鼻腔非特异性免疫的重要结构,鼻黏膜表面黏液清除的动力来自纤毛微管蛋白臂的滑动。研究发现空气污染、长期吸烟及哮喘等环境或疾病影响时,纤毛超微结构受损、摆动能力下降、 摆动方向紊乱以及黏附其表面黏液毯的能力下降,最终表现为清除功能障碍[9]。本研究模型组观察到大量短小纤毛穿插于正常纤毛之间,其直径约0.08~0.12 μm,长约1~4 μm,含零至数个微管,或微管排列乱而计数不清、无九组二联结构,以仅含一组中央微管者多见。Toskala等[10]发现变应性鼻炎鼻黏膜中短纤毛明显增多。纤毛的有效摆动依靠完整的微管二联体之间的滑动而实现,而本研究中变应性鼻炎大鼠鼻黏膜短小纤毛内无微管或仅一组中央微管。且纤毛长度需高于其周围黏液层的厚度方可发挥有效清除作用[11],正常纤毛长度5~8 μm,而短小纤毛长度小于5 μm,只能浸润于黏液毯的水样层,接触不到外层的凝胶层,故认为短小纤毛无有效的黏液清除功能。本研究中,AR模型组于干预2周时观察到异常增厚呈条带状、大片状黏附于纤毛顶端的黏胶层,其内含有中性粒细胞、单核细胞、嗜酸性粒细胞等炎性细胞,纤毛粘连成簇。在气道变应性疾病中,黏膜上皮杯状细胞化生,黏膜下黏液性和浆液性腺体增生,黏液分泌增加,黏液层增厚乃至超过纤毛的承载量,且其黏滞性增加,最终使得纤毛出现无效运动,黏液毯滞留于纤毛顶端,纤毛清除功能下降。而黏液纤毛系统清除功能障碍又导致短小纤毛出现率增加[10],进一步阻碍清除功能,进而出现纤毛倒伏、脱落等进行性损害。结合本研究中短纤毛的数量在不同分组中及不同时间点的差异,认为短小纤毛的数量可作为判断变应性鼻炎鼻黏膜病变程度的参考之一。随着变应原的持续刺激,短小纤毛先增多后减少:发病急性期黏液分泌增多,纤毛清除功能下降,促使鼻黏膜损伤修复、短小纤毛增多;随着疾病进展,细胞损害加重,细胞器溶解,细胞膜破裂、细胞膜上纤毛进一步脱落,短小纤毛亦减少,加之杯状细胞大量化生,细胞外基质沉积,基底膜增厚,鼻黏膜进入重塑后的不可逆病损阶段。
GA为五环三萜皂苷类化合物,具有类激素样作用,研究发现18β-GA可使HSP90与糖皮质激素受体的结合发生解离,从而抑制炎症反应[12]。而糖皮质激素对变应性鼻炎有抗炎、减轻水肿及修复鼻黏膜上皮等作用。Bodet等[13]报道,甘草提取物可抑制脂多糖(LPS)诱导的体外培养巨噬细胞分泌IL-1β、IL-6、IL-8、TNF-α及其他炎性细胞因子。而Kim等[14]以甘草的乙醇提取物干预LPS小鼠急性炎症模型,发现甘草提取物不仅可降低IL-6、TNF-α等炎性因子的水平,还可在一定程度上升高IL-10等抑炎因子水平。Matsui等[15]研究发现人肺成纤维细胞具有产生炎症因子IL-8 和嗜酸细胞活化趋化因子的潜能,而甘草酸和其衍生物具有降低成纤维细胞炎症活化因子IL-8和嗜酸细胞活化趋化因子水平的作用。Shin等[16]报道18β-GA对DNP-HAS刺激体外培养的RBL-2H3细胞所致的IgE升高起到抑制作用,并能有效抑制腹腔注射化合物48/80诱导的被动皮肤过敏反应小鼠模型肥大细胞脱颗粒反应、明显减轻小鼠搔抓行为。 Ma等[17]以OVA 刺激建立变应性哮喘小鼠模型,发现经GA干预后哮喘小鼠血清Th2细胞因子IL-4、IL-5及IL-13水平提高,Th1细胞因子IFN-γ水平下降,气道嗜酸粒细胞浸润减少。上述研究均不同程度地提示甘草次酸对变态反应性疾病中IgE及多种炎性因子具有调节作用,有理由推测甘草次酸对于变应性鼻炎的潜在治疗价值。
本研究通过观察变应性鼻炎大鼠经GA干预后鼻部症状及鼻黏膜超微结构变化,初步直观地了解GA对变应性鼻炎的鼻黏膜病理改变的影响,发现在18β-GA干预过程中,变应性鼻炎大鼠搔鼻、喷嚏次数及流涕逐渐减少,在干预2周后症状叠加评分与模型组差异有统计学意义。此后的干预过程中,18β-GA组大鼠行为学表现趋于稳定,偶有搔鼻、喷嚏动作,外鼻无清涕;电镜下纤毛的超微结构表现为一定程度上逐步改善:干预2周后鼻黏膜纤毛较密集、局部有簇状粘连,“9×2+2”微管结构完整,氯雷他定组除纤毛束状粘连外还可见增厚的黏液毯呈细条带状覆于纤毛顶端,但面积小于模型组;干预4周后18β-GA组与氯雷他定组表现相似:密集的纤毛中局部束状粘连较2周时减少,透射电镜下纤毛呈规则的“9×2+2”微管结构;至6周时,18β-GA组及氯雷他定组纤毛密集程度接近空白组,但局部纤毛排列散乱,偶见纤毛顶端粘连呈细束;至10周时,18β-GA组和氯雷他定组纤毛密集、无明显粘连,局部纤毛排列略散乱,透射电镜下纤毛微管结构完整。因此,基于18β-GA干预后动物的行为学表现及鼻黏膜纤毛超微结构变化,初步认为18β-GA能有效减轻变应性鼻炎大鼠的行为学表现、修复损伤的鼻黏膜、促进纤毛增生,减轻鼻黏膜病理学改变。但GA在变应性鼻炎疾病模型中的作用机制以及相关的信号途径等仍需进一步研究。 4 利益冲突
所有作者声明本文不涉及任何利益冲突。
| [1] | Bro ek J L, Bousquet J, Baena-Cagnani C E, Bonini S, Canonica G W, Casale T B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision[J].J Allergy Clin Immunol,2010, 126: 466-476. |
| [2] | Ming L J, Yin A C. Therapeutic effects of glycyrrhizic acid[J].Nat Prod Commun,2013, 8: 415-418. |
| [3] | Wang C Y, Kao T C, Lo W H, Yen G C. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions[J].J Agric Food Chem,2011, 59: 7726-7733. |
| [4] | 李 钦, 李玉芬, 陈彦林, 张大良, 刘言训. 白细胞介素5和13 受体对变应性鼻炎大鼠血管细胞黏附分子1及γ干扰素的影响[J].中华耳鼻咽喉头颈外科杂志,2012, 47: 638-641. |
| [5] | Guibas G V, Spandou E, Meditskou S, Vyzantiadis T A, Priftis K N, Anogianakis G. N-acetylcysteine exerts therapeutic action in a rat model of allergic rhinitis[J]. Int Forum Allergy Rhinol, 2013,3:543-549. |
| [6] | Ogawa T, Shimizu S, Shimizu T. The effect of heparin on antigen-induced mucus hypersecretion in the nasal epithelium of sensitized rats[J]. Allergol Int, 2013,62:77-83. |
| [7] | 韩 菲, 安云芳, 赵长青.大鼠变应性鼻炎模型下呼吸道细胞因子及黏蛋白的改变[J].中华耳鼻咽喉头颈外科杂志,2005, 40:339-342. |
| [8] | 刘建国, 刘月辉. 变应性鼻炎大鼠鼻黏膜纤毛超微结构和鼻症状变化的研究[J].临床耳鼻咽喉头颈外科杂志,2010,24:365-368. |
| [9] | Houtmeyers E, Gosselink R, Gayan-Ramirez G, Decramer M. Regulation of mucociliary clearance in health and disease[J]. Eur Respir J,1999, 13: 1177-1188. |
| [10] | Toskala E, Nuutinen J, Rautiainen M, Torkkeli T.The correlation of mucociliary transport and scanning electron microscopy of nasal mucosa[J].Acta Otolaryngol,1995, 115: 61-65. |
| [11] | Konrad F, Schiener R, Marx T, Georgieff M.Ultrastructure and mucociliary transport of bronchial respiratory epithelium in intubated patients[J]. Intensive Care Med,1995, 21: 482-489. |
| [12] | Kao T C, Shyuand M H, Yen G C.Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation[J].J Agric Food Chem,2010, 58: 8623-8629. |
| [13] | Bodet C, La V D, Gafner S, Bergeron C, Grenier D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood[J].J Periodontol,2008,79: 1752-1761. |
| [14] | Kim J K, Oh S M, Kwon H S, Oh Y S, Lim S S, Shin H K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages[J]. Biochem Biophys Res Commun,2006, 345:1215-1223. |
| [15] | Matsui S, Matsumoto H, Sonoda Y, Ando K, Aizu-Yokota E, Sato T, et al. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line[J].Int Immunopharmacol,2004, 4:1633-1644. |
| [16] | Shin Y W, Bae E A, Lee B, Lee S H, Kim J A, Kim Y S. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components[J].Planta Med,2007,73: 257-261. |
| [17] | Ma C, Ma Z, Liao X L, Liu J, Fu Q, Ma S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4+CD25+Foxp3+ regulatory T cells in ovalbumin-sensitized mice[J].J Ethnopharmacol,2013,148:755-762. |
 2015, Vol. 36
2015, Vol. 36


