脊柱骨折常伴发严重的脊髓损伤,导致效应器官及组织失神经营养发生功能紊乱,严重损害患者身心健康。因此,保护脊髓损伤后残存神经元功能具有重要的临床意义。心脏营养素-1(cardiotrophin-1,CT-1)是白细胞介素-6(IL-6)家族的新成员[ 1],在小鼠心脏、肾脏、骨骼肌和肝脏中均有表达[ 2],也表达于小鼠胚胎脑、脊髓、背根神经节、骨骼肌和皮肤中[ 3],在交感神经轴突损伤中高度表达其受体gp130[ 4],能够支持脊髓运动神经元、多巴胺能神经元和睫状神经节神经元长期存活[ 5,6],具有潜在的神经保护作用。但目前缺乏其对脊髓损伤后神经保护及再生作用的相关研究。因此,本研究观察大鼠脊髓损伤组织中CT-1及其受体的表达变化,并进一步观察外源性重组CT-1对损伤脊髓神经元修复的可能作用,探讨CT-1在脊髓损伤修复中的潜在价值,为其在脊髓损伤中的临床应用提供参考。
1 材料和方法 1.1 主要试剂及仪器rhCT-1(Peprotech,美国)、苏木精-伊红(H-E)染色试剂盒(北京雷根生物技术有限公司)、尼氏(Nissl)染色试剂盒(南京森贝伽生物科技有限公司)、反转录反应液(美国e-life Co公司)、实时荧光定量PCR仪(CFX ConnectTM,Bio-Rad,美国)、计算机图像分析系统(Olympus,BX51WI-DPMC,Japan)、酶标仪(Biotek公司,美国)。
1.2 动物来源及脊髓损伤模型的建立雄性SD大鼠由第二军医大学动物实验中心提供,6个月龄,体质量200~250 g。脊髓损伤模型的建立:大鼠称质量,腹腔注射0.5%硫喷妥钠(500 mg/kg)麻醉后,俯卧固定,备皮,常规消毒铺巾。以脊柱为中心纵行切开皮肤、皮下组织,剥离两侧椎旁肌肉,暴露T6~8棘突和椎板,咬除T7棘突和椎板,暴露硬膜,预打击脊髓上放置0.4 cm×0.5 cm垫片。采用Allen打击法[ 7](10 g×5 cm),以造成截瘫为有效打击,关闭伤口,行椎管内置管术。术后室温控制在20~28℃,所有动物每日肌注青霉素钠10万U。
1.3 RT-PCR测定大鼠脊髓损伤组织CT-1及其受体的表达健康雄性SD大鼠90只,按照取材时间点随机分为9组:脊髓损伤前正常大鼠(0 h)以及大鼠脊髓损伤模型建立后1 h、6 h、12 h、1 d、2 d、3 d、6 d、12 d组(n=10),每只大鼠取损伤处脊髓组织50 mg,采用RT-PCR方法检测CT-1及其受体mRNA表达。CT-1引物序列:5′-GGT GTG TTG AAG GAA ACA GG-3′(Forward),5′-GAG TCA GTC TGG TGG TCT TCC-3′(Reverse);糖蛋白130(gp130):5′-GCA AGG TGT TTG CTG AAG GG-3′(Forward),5′-ACG GCT TCG TCA GCT TTG TA-3′(Reverse);白血病抑制因子受体(LIFR-α):5′-GGA CTG ACT GAA TAG CAC GGA-3′(Forward),5′-CAA TGC CTG GGT ACA TGG TAG-3′(Reverse)。以GAPDH管家基因为内参,根据ΔCt值比较平均相对表达量的变化。RT-PCR检测委托上海舜百生物科技有限公司完成。
1.4 外源性CT-1对损伤脊髓神经元的影响雄性SD大鼠90只,随机分为3组:模型组、神经生长因子(NGF)干预组和CT-1干预组(n=30)。CT-1干预组于脊髓损伤后即刻予rhCT-1(100 μg·kg-1·d-1)硬膜内灌注,模型组、NGF干预组分别给予等量生理盐水和NGF。给药后第3天、1周、2周于损伤脊髓处取材,行H-E染色及Nissl染色,通过计算机图像分析系统(Olympus,BX51WI-DPMC,Japan)于放大200倍显微镜视野中进行计数分析。
1.5 外源性CT-1对脊髓损伤后失神经营养肌肉的影响雄性SD大鼠60只,随机分为模型组及CT-1干预组(n=30),所有大鼠均制备脊髓损伤模型,CT-1干预组大鼠于脊髓损伤后即刻给予rhCT-1(100 μg·kg-1·d-1)硬膜内灌注,损伤后1、4、12周完整剥离大鼠小腿三头肌(n=10),去除脂肪及结缔组织,滤纸吸干表面血液后迅速转送至电子分析天平(R200D,1/10万,Germany)上称质量。取上述剥离肌肉行总蛋白测定:将肌肉组织于冰上切碎,按试剂盒(美季生物公司)说明应用Lowry法测定总蛋白。
1.6 统计学处理采用SPSS 19.0分析软件,应用方差分析及t检验比较组间差异,检验水准(α)为0.05。
2 结 果 2.1 大鼠脊髓损伤组织CT-1及其受体表达的变化结果(图 1)表明:损伤脊髓组织中CT-1 mRNA的表达出现波动,伤后1 h相对表达量显著增高(P<0.05);随后其进行性下降,损伤后24 h与0 h表达水平相当,随时间的延长呈现低表达表现。其受体gp130、LIFR的表达出现不同程度的变化,前者12 h内表达平稳,与0 h相当,48 h后表达水平逐渐升高,6 d出现峰值(P<0.05),12 d后降至0 h水平;后者于48 h时形成表达高峰(P<0.05),72 h时略有下降,6 d后与0 h表达水平相当。
 | 图 1 大鼠损伤脊髓组织CT-1及其受体基因表达水平的变化Fig. 1 Changes of CT-1,gp130,and LIFR mRNA expression in injured spinal cord tissues of rats*P<0.05 vs 0 h; n=10,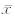 ±s ±s |
H-E染色结果(图 2A)表明:NGF及CT-1干预组损伤脊髓神经元计数均高于模型组,差异有统计学意义(P<0.05),前两组间差异无统计学 意义(图 2B)。Nissl染色结果(图 3A)表明:NGF及CT-1干预组损伤脊髓神经元计数均高于模型组,差异有统计学意义(P<0.05),前两组间差异无统计学意义(图 3B)。
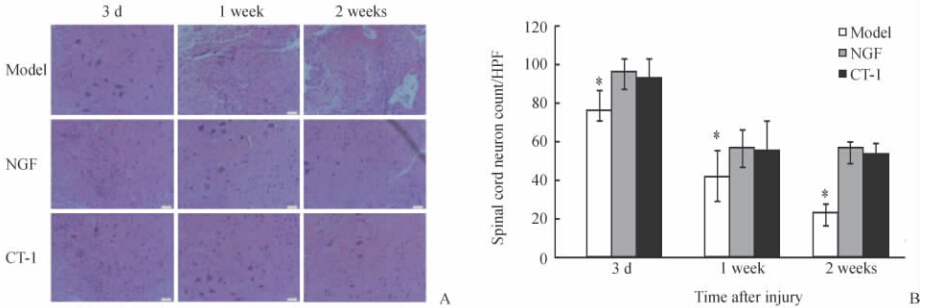 | 图 2 不同组别大鼠损伤脊髓神经元H-E染色(A)及神经元计数(B)Fig. 2 H-E staining(A) and spinal cord neuron count(B) of damaged rat spinal cord in three groups at different time pointsA: As time went by the number of neuron further decreased in model group and fibrosis was observed in the injuried spinal tissues,but there was no significant difference between NGF and CT-1 group.
Original magnification:×200. B: Spinal cord neuron count per high power field (HPF). *P<0.05 vs NGF or CT-1 treatment group; n=10,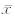 ±s ±s |
 | 图 3 不同组别大鼠损伤脊髓神经元Nissl染色(A)及尼氏体计数(B)Fig. 3 Nissl staining(A) and Nissl bodies count(B) of damaged rat spinal cord in three groups at different time pointsA: Fibroblasts were increased in the model group 2 weeks after damaging.
Original magnification:×200. B: Nissl body count per high power field (HPF). *P<0.05 vs NGF or CT-1 treatment group; n=10,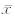 ±s ±s |
2.3 外源性CT-1干预对大鼠失神经营养肌肉的影响
脊髓损伤后,大鼠肌肉因失神经营养作用,表现为灰白、弹性差;比较两组肌肉湿质量,结果显示:在损伤后4周、12周时,CT-1组肌肉湿质量、总蛋白含量均高于模型组(P<0.05),1周时差异无统计意义(图 4)。
 | 图 4 脊髓损伤后不同组别大鼠小腿三头肌肌肉湿质量(A)及肌蛋白含量(B)的比较Fig. 4 Muscle wet weight(A) and the protein contents(B) of rat triceps of different groups at different time points after spinal cord injury*P<0.05 vs model group; n=10,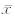 ±s ±s |
3 讨 论
近年来,随着我国经济的高速发展及基础设施的不断完善,交通意外的发生不断提高,脊髓损伤患者也逐年增加,严重威胁国人健康和生命[ 8]。目前临床上对脊髓损伤的治疗疗效均不理想,是困扰医学界的难题之一[ 9,10,11]。CT-1在神经系统中是一种重要的调节因子,可以促进交感神经元、运动神经元、感觉神经元的存活[ 4]。不同分化程度的骨骼肌细胞来源的CT-1均可以支持脊髓运动神经元、多巴胺能神经元和睫状神经节神经元的长期存活[ 12],CT-1还能刺激体外培养的胎儿运动神经元的存活[ 13]。在对小鼠特发性神经元疾病中也证实了CT-1对神经元的保护性作用[ 14]。这些研究成果证实,CT-1作为神经活性因子为脊髓损伤实验开展提供了良好的基础。CT-1还可降低氧自由基诱导剂FeSO4和神经毒性物质SIN-1诱导的皮质神经元细胞的死亡[ 15]。
本研究结果表明:CT-1干预组与NGF干预组神经元、尼氏体数量差异无统计学意义,均高于模型组;且随着时间的延长,两者保护效果更为显著。NGF是传统的神经生长因子,对脊髓损伤后神经元结构重塑、功能恢复具有确切的疗效[ 16]。本研究提示外源性CT-1对损伤脊髓神经元形态、数量等的保护作用已接近NGF。CT-1亦可促进髓鞘细胞的生长,为神经纤维的再生爬行提供了良好的内环境支撑[ 17]。本研究的形态学观察结果表明CT-1在对抗损伤脊髓神经元死亡的作用上与传统神经生长因子大致相当。需要指出的是,通过观察切片发现,脊髓损伤2周后CT-1组神经元数量较损伤3 d仍有减少,提示继发性损伤因素仍然存在。相比模型组,CT-1可以部分减缓这种损伤。既往研究表明复合使用不同种类神经营养因子可以有效提高去神经化肌肉质量[ 18],而本研究结果表明损伤后4周、8周时CT-1干预组肌肉湿质量高于模型组,初步推测其对于肌肉功能的支持保护作用同复合神经营养因子相当。脊髓损伤后CT-1 mRNA表达出现一过性增高,继而出现持续性下降。此过程提示:正常脊髓组织中CT-1及其受体亚基表达相对恒定,脊髓损伤后,损伤部位充血水肿,并产生大量炎症介质,结合外周组织中被趋化的炎症介质,共同组成了CT-1的高峰;随着炎症反应减弱,CT-1的表达出现降低。同时,其受体亚基mNRA表达相应上调,以增加对CT-1的敏感性,与既往研究类似[ 19]。本实验结果显示,损伤后48 h~6 d,CT-1受体达高峰,故对脊髓损伤患者开展早期临床干预具有重要意义。当然,CT-1受体在体内多个器官、组织存在,故对用药方式及治疗的安全剂量方面仍需进一步探讨[ 20]。
综上所述,CT-1对脊髓损伤后神经死亡进程起到了一定的减缓作用,为神经轴突的爬行重建提供了良好的环境,可能对脊髓功能的保护起到了一定的帮助作用,值得深入研究。
4 利益冲突
所有作者声明本文不涉及任何利益冲突。
| [1] | Pennica D,King K L,Shaw K J,Luis E,Rullamas J,Luoh S M,et al.Expression cloning of cardiotrophin 1,a cytokine that induces cardiac myocyte hypertrophy[J].Proc Natl Acad Sci USA,1995,92:1142-1146. |
| [2] | Kanazawa H,Ieda M,Kimura K,Arai T,Kawaguchi-Manabe H,Matsuhashi T,et al.Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents[J].J Clin Invest,2010,120:408-421. |
| [3] | Jin H,Yang R,Keller G A,Ryan A,Ko A,Finkle D,et al.In vivo effects of cardiotrophin-1[J].Cytokine,1996,8:920-926. |
| [4] | Habecker B A,Sachs H H,Rohrer H,Zigmond R E.The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons[J].Dev Neurobiol,2009,69:392-400. |
| [5] | Arce V,Garces A,de Bovis B,Filippi P,Henderson C,Pettmann B,et al.Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique[J].J Neurosci Res,1999,55:119-126. |
| [6] | Latchman D S.Cardiotrophin-1:a novel cytokine and its effects in the heart and other tissues[J].Pharmacol Ther,2000,85:29-37. |
| [7] | Allen A.Surgery for experimental lesions of spinal cord equivalent to crush injury of fracture dislocation of spinal column:a preliminary report[J].JAMA,1911,57:878-880. |
| [8] | 李建军,周红俊,洪毅.2002年北京市脊髓损伤发病率调查[J].中国康复理论与实践,2004,10:412-413. |
| [9] | Bamford J A,Mushahwar V K.Intraspinal microstimulation for the recovery of function following spinal cord injury[J].Prog Brain Res,2011,194:227-239. |
| [10] | Tate D G,Boninger M L,Jackson A B.Future directions for spinal cord injury research:recent developments and model systems contributions[J].Arch Phys Med Rehabil,2011,92:509-515. |
| [11] | Ito Z,Sakamoto K,Imagama S,Matsuyama Y,Zhang H,Hirano K,et al.N-acetylglucosamine 6-O-sulfotransferase-1-deficient mice show better functional recovery after spinal cord injury[J].J Neurosci,2010,30:5937-5947. |
| [12] | Pennica D,Arce V,Swanson T A,Vejsada R,Pollock R A,Armanini M,et al.Cardiotrophin-1,a cytokine present in embryonic muscle,supports long-term survival of spinal motoneurons[J].Neuron,1996,17:63-74. |
| [13] | Lesbordes J C,Cifuentes-Diaz C,Miroglio A,Joshi V,Bordet T,Kahn A,et al.Therapeutic benefits of cardiotrophin-1 gene transfer in a mouse model of spinal muscular atrophy[J].Hum Mol Genet,2003,12:1233-1239. |
| [14] | Mitsumoto H,Klinkosz B,Pioro E P,Tsuzaka K,Ishiyama T,O’Leary R M,et al.Effects of cardiotrophin-1 (CT-1) in a mouse motor neuron disease[J].Muscle Nerve,2001,24:769-777. |
| [15] | Wen T C,Rogido M R,Moore J E,Genetta T,Peng H,Sola A.Cardiotrophin-1 protects cortical neuronal cells against free radical-induced injuries in vitro[J].Neurosci Lett,2005,387:38-42. |
| [16] | Hollis E R 2nd,Tuszynski M H.Neurotrophins:potential therapeutic tools for the treatment of spinal cord injury[J].Neurotherapeutics,2011,8:694-703. |
| [17] | 辛泽团,刘湘泽,张明进,李伟,陈伯华,马学晓.外源性心脏营养素1:保护PC12细胞和许旺细胞的可能性[J].中国组织工程研究,2013,17:7265-7271. |
| [18] | Chen J,Chu Y F,Chen J M,Li B C.Synergistic effects of NGF,CNTF and GDNF on functional recovery following sciatic nerve injury in rats[J].Adv Med Sci,2010,55:32-42. |
| [19] | 马学晓,张高孟,冯勇,顾玉东,顾建新.心脏营养素-1 对失神经骨骼肌的营养和保护作用[J].中华手外科杂志,2006,22:112-115. |
| [20] | Bordet T,Castelnau-Ptakhine L,Fauchereau F,Friocourt G,Kahn A,Haase G.Neuronal targeting of cardiotrophin-1 by coupling with tetanus toxin C fragment[J].Mol Cell Neurosci,2001,17:842-854. |
 2014, Vol. 35
2014, Vol. 35


