2. 第二军医大学长海医院病理科, 上海 200433
2. Department of Pathology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
表皮生长因子受体(epidermal growth factor receptor,EGFR)是肿瘤靶向治疗的重要靶标,其酪氨酸激酶抑制剂如吉非替尼、厄洛替尼等治疗非小细胞肺癌(non-small cell lung cancer, NSCLC)尤其是EGFR敏感突变的肺腺癌(adenocarcinoma,ADC)具有显著疗效[1 ,2]。EGFR基因突变率在东亚裔ADC人群中为30%~40%,但在同地区其他组织类型的NSCLC中则较低[3 ,4],因此目前临床上对肺鳞癌(squamous carcinoma,SQC)患者检测EGFR突变的意义尚存争议[5]。
p63是抑癌基因p53家族成员之一,由于启动子不同和3′端剪切方式不同, p63基因可编码多种具有不同活性的异构体。这些异构体主要分为具有反式激活区的TA异构体和N末端截短的ΔNp63α异构体,前者具有p53样活性,可诱导细胞周期停滞和凋亡,后者则抑制p53的功能,促进转化细胞的生长[6,7,8]。我们先前的研究表明,肺SQC中高表达p63,且其表达水平与组织学或细胞学的采样方法以及分化程度无关[9]。本研究检测了EGFR在肺SQC患者中的突变率并分析了p63对EGFR突变肺SQC患者生存的影响。 1 材料和方法 1.1 样本采集
收集2010年2月至2013年3月经病理组织学确诊的初治肺SQC样本。样本来自于经CT引导的细针活检、支气管镜下经支气管的活检或者手术切除样本。 1.2 EGFR基因突变检测
采用直接测序法对EGFR的4个位点(18~21位点)进行检测[10]。应用PCR直接扩增EGFR 18~21位点的基因片段。2%琼脂糖凝胶电泳鉴定扩增片段,通过凝胶成像系统观察PCR产物电泳结果。PCR产物进行正反两个方向测序。分析测序图谱,判断EGFR基因18~21位点外显子区域是否存在突变。 1.3 p63表达检测
主要试剂抗人p63单克隆抗体及EnVision两步法试剂盒均为丹麦DAKO公司产品。组织均经4%中性甲醛溶液固定,常规脱水后石蜡包埋,切成3~4 μm厚的薄片,在乙二胺四乙酸溶液(EDTA,pH 8.0)中进行热诱导抗原修复。然后用3%过氧化氢阻断内源性过氧化氢酶活性,加入抗人p63单克隆抗体(clone 4A4)作为标记,EnVision两步法染色,显微镜下观察。结果判断: 细胞表现出一种弥漫染色或者平均阳性染色面积≥10%定义为免疫反应阳性(+);细胞染色面积介于1%和9%之间定义为免疫反应部分阳性(部分+);细胞完全没有被染色定义为免疫反应阴性(-)[11]。 1.4 生存评价
以无疾病进展时间(progression-free survival,PFS)和总生存时间(overall survival,OS)作为生存评价指标。PFS:从确诊疾病到影像学上第1次发生疾病进展或其他任何原因导致死亡的时间;OS:从确诊开始到死亡或末次随访的时间。 1.5 统计学处理
用Kaplan-Meier法估计生存时间,log-rank法分析生存差异。检验水准(α)为0.05。 2 结 果 2.1 肺SQC患者EGFR突变率
共收取267例经病理组织学确诊的肺SQC样本,经2位富有经验的病理学专家复检,排除5例鳞腺混合样本,最终262例样本纳入研究。262例样本中,16例检测到EGFR基因突变,突变率为6.1%。其中外显子19缺失7例(43.8%),外显子21点突变(L858R)9例(56.2%),未检测到外显子18和20突变。 2.2 EGFR突变肺SQC患者的临床病理特征
由表 1可见,16例EGFR突变肺SQC患者女性比例和非吸烟者比例较非突变患者高(P<0.05)。由表 2可见,所有16例EGFR突变患者中,13例(81.3%)为ⅢB或 Ⅳ期肺SQC,其中3例(23.1%)是女性,7例(53.8%)是非吸烟者。
| 表 1 EGFR突变与非突变肺SQC患者的临床特征比较Tab 1 Comparison of clinical data between EGFR mutation and non-EGFR mutation in lung SQC patients |
| 表 2 EGFR突变肺SQC患者一般资料、突变类型、 临床病理特征以及生存时间汇总 Tab 2 Clinical data, mutation types, pathologic findings, and survival time of EGFR-mutated lung SQC patients |
262例样本中,p63阳性(图 1A)247例,p63部分阳性(图 1B)10例,p63阴性5例。p63部分阳性和阴性的15例均由病理学专家再次复核证实为肺SQC。16例EGFR突变样本的p63表达情况见表 2。
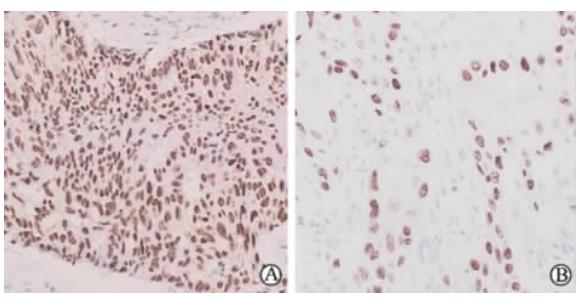 | 图 1 p63在肺SQC中不同免疫活性的表达 Fig 1 Different immunoreactivities of p63 in lung SQC patients SQC: Squamous carcinoma. A: p63 positive sample; B: p63 partially positive sample. Original magnification: ×400 |
截至2013年5月20日,16例EGFR突变患者无一例失访。随访时间5.23~ 35.93 个月,中位随访时间13.90(95% CI: 8.76~19.04)个月。16例EGFR突变患者中15例(93.8%)疾病进展,进展患者中11例(68.8%)已死亡。16例EGFR突变患者的PFS和OS见表 2。
根据p63免疫反应活性,把16例EGFR突变患者分成3组: p63阴性组1例, p63部分阳性组4例, p63阳性组11例。p63阴性组因只有1例而未进行统计学分析。4例p63部分阳性患者中位PFS为7.76(95% CI:3.12~12.34)个月,中位OS为11.28(95%CI: 9.33~15.23)个月。11例p63阳性患者中位PFS为4.43(95% CI:2.65~6.22)个月,与p63部分阳性患者相比差异无统计学意义(P=0.256);中位OS为5.93(95%CI: 1.75~8.39)个月,与p63部分阳性患者相比差异有统计学意义(P=0.039,图 2)。
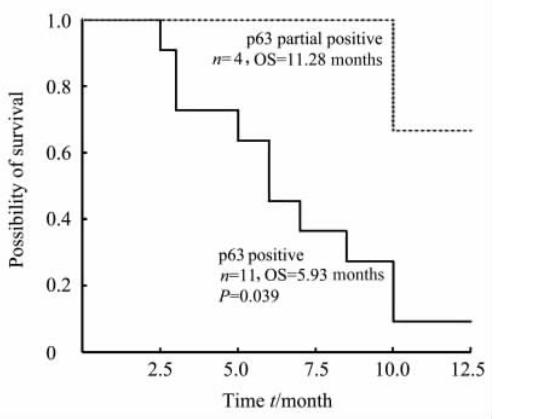 | 图 2 p63免疫活性与肺SQC EGFR突变患者生存的关系 Fig 2 Relationship between p63 immunoreactivity andsurvival of SQC patients with EGFR mutation SQC: Squamous cell carcinoma; EGFR: Epidermal growth factor receptor; OS: Overall survival |
EGFR在SQC中的突变率文献报道各不相同。Mu等[12]报道肺SQC的EGFR突变率为5.9%(6/102),而Lai等[13]报道282例肺SQC中有41例EGFR突变,突变率为14.5%。然而Hata等[14]研究认为,纯SQC无EFGR突变。他们对原有测得的33例EGFR突变的肺SQC(33/249)进行复检并结合p63、TTF-1等免疫组化指标检测,结果显示这33例EGFR突变肺SQC都混有不同程度ADC成分,因此认为EGFR在肺SQC中的突变是由于肺SQC中混有ADC成分所致。但大多数学者都认为肺SQC确实存在EGFR突变,本研究结果也与此一致。本研究发现,在262例初治肺SQC样本中有16例EGFR基因敏感突变,突变率为6.1%。根据现有结果,我们认为EGFR突变确实发生在肺SQC患者中,EGFR是肺SQC的驱动基因之一。
与传统意义上的肺SQC患者相比,EGFR突变患者的临床特征显示有更多的女性(37.5% vs 3.7%)和非吸烟者(62.5% vs 26.0%),提示EGFR突变的肺SQC患者可能与传统的肺SQC患者有着不同的遗传背景。本研究中EGFR突变类型以及各类型百分比(外显子19缺失,43.8%;L858R,56.2%),与其他作者[3,12-13]研究结果大致相同。有文献报道EGFR基因在SQC中存在非敏感位点突变,包括外显子21 N826S、A859T、L861Q、V843I、K860E等 [15,16,17,1819],但报道呈现单个、非重复性。因此,对于检测敏感位点L858R和外显子19缺失以外的非敏感位点,是不必要和不经济的。
p63是免疫组化诊断肺SQC的常用指标,本研究发现p63与EGFR之间似乎对肺SQC患者生存有影响:p63部分阳性EGFR突变患者的OS为11.28(95%CI: 9.33~15.23)个月,比p63阳性EGFR突变患者的OS (5.93个月,95%CI: 1.75~8.39个月)更长(P=0.039)。p63和EGFR两者的关系可能与胰岛素样生长因子结合蛋白3(IGFBP-3)相关。IGFBP-3是EGFR和p63下游信号转导的共同产物。EGFR能够活化下游信号转导通路,其中包括磷脂酰肌醇3激酶/蛋白激酶B (PI3K/Akt)通路,它能够刺激细胞增殖以及阻止肿瘤细胞凋亡[20]。IGFBP-3作为PI3K/Akt的转录产物,主要起到阻止肿瘤细胞凋亡的作用,从而促进肿瘤的生长繁殖[21]。而p63亚基之一的ΔNp63α同样能激活下游信号转导通路PI3K/Akt,ΔNp63α介导的转录产物IGFBP-3可使过度表达p63的肿瘤细胞免于凋亡[6]。由此推测,检测SQC细胞中IGFBP-3的含量可以评估EGFR和p63的表达活性,进而更好地评价患者的生存状况。
p63免疫活性与肺SQC患者生存关系的报道较少,结论不一。Pelosi等[22]报道p63的免疫活性与患者OS和PFS无关,而Massion等[23]指出肺SQC患者中p63的免疫强度与患者生存时间呈正相关,免疫染色强度越高越有利于患者的生存。我们对EGFR突变肺SQC患者进行生存分析,结果发现p63部分阳性的 EGFR突变患者比p63阳性EGFR突变患者生存期更长,提示p63免疫强度与EGFR突变肺SQC患者生存时间呈负相关。由于EGFR突变样本例数较少,且生存分析结果受其他多重因素综合影响,本研究结果还需要更大样本来进行验证。 4 利益冲突
所有作者声明本文不涉及任何利益冲突。
| [1] | Lee V W, Schwander B, Lee V H.Effectiveness and cost-effectiveness of erlotinib versus gefitinib in first-line treatment of epidermal growth factor receptor-activating mutation-positive non-small-cell lung cancer patients in Hong Kong[J].Hong Kong Med J, 2013, 10:2129-2139. |
| [2] | Keam B, Kim D W, Park J H, Lee J O, Kim T M, Lee S H, et al.How molecular understanding affects to prescribing patterns and clinical outcome of gefitinib in non-small cell lung cancer? 10 year experience of single institution[J].Cancer Res Treat, 2013, 45:178-185. |
| [3] | Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Seto T, et al.Updated overall survival results of WJTOG 3405, a randomized phase Ⅲ trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR)[J].J Clin Oncol, 2012, 30 (Suppl):Abstr 7521. |
| [4] | Mok T S, Wu Y L, Yu C J, Zhou C, Chen Y M, Zhang L, et al.A randomized placebo-controlled phase Ⅲ study of intercalated erlotinib with gemcitabine/platinum in first-line advanced non-small cell lung cancer (NSCLC):FASTACT-Ⅱ[J].J Clin Oncol, 2012, 30 (Suppl):Abstr.7520. |
| [5] | Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba I I, et al.Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers[J].J Natl Cancer Inst, 2005, 97:339-346. |
| [6] | Chen X.The p53 family:same response, different signals?[J].Mol Med Today, 1999, 5:387-392. |
| [7] | Flores E R, Tsai K Y, Crowley D, Sengupta S, Yang A, McKeon F, et al.p63 and p73 are required for p53-dependent apoptosis in response to DNA damage[J].Nature, 2002, 416:560-564. |
| [8] | Di Como C J, Urist M J, Babayan I, Drobnjak M, Hedvat C V, Teruya-Feldstein J, et al.p63 expression profiles in human normal and tumor tissues[J].Clin Cancer Res, 2002, 8:494-501. |
| [9] | Wang B, Han Y, Zang J.Expression of p63 in squamous cell carcinoma of the lung and its diagnostic significance:a meta-analysis[J].J Cancer Res Updates, 2012, 1:228-238. |
| [10] | Lynch T J, Bell D W, Sordella R, Gurubhagavatula S, Okimoto R A, Brannigan B W, et al.Activating mutations in the epidermal growth factor receptor underlying responsiveness of non small-cell lung cancer to gefitinib[J].N Engl J Med, 2004, 350:2129-2139. |
| [11] | Yanagita E, Imagawa N, Ohbayashi C, Itoh T.Rapid multiplex immunohistochemistry using the 4-antibody cocktail YANA-4 in differentiating primary adenocarcinoma from squamous cell carcinoma of the lung[J].Appl Immunohistochem Mol Morphol, 2011, 19:509-513. |
| [12] | Mu X L, Li L Y, Zhang X T, Wang M Z, Feng R E, Cui Q C, et al.Gefitinib-sensitive mutations of the epidermal growth factor receptor tyrosine kinase domain in Chinese patients with non-small cell lung cancer[J].Clin Cancer Res, 2005, 11:4289-4294. |
| [13] | Lai Y, Zhang Z, Li J, Sun D, Zhou Y, Jiang T, et al.EGFR mutations in surgically resected fresh specimens from 697 consecutive Chinese patients with non-small cell lung cancer and their relationships with clinical features[J].Int J Mol Sci, 2013, 14:24549-24559. |
| [14] | Hata A, Katakami N, Yoshioka H, Kunimasa K, Fujita S, Kaji R, et al.How sensitive are epidermal growth factor receptor-tyrosine kinase inhibitors for squamous cell carcinoma of the lung harboring EGFR gene-sensitive mutations?[J].J Thorac Oncol, 2013, 8:89-95. |
| [15] | Sugio K, Uramoto H, Ono K, Oyama T, Hanagiri T, Sugaya M, et al.Mutations within the tyrosine kinase domain of EGFR gene specifically occur in lung adenocarcinoma patients with a low exposure of tobacco smoking[J].Br J Cancer, 2006, 94:896-903. |
| [16] | Mitsudomi T, Yatabe Y.Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer[J].Cancer Sci, 2007, 98:1817-1824. |
| [17] | Chou T Y, Chiu C H, Li L H, Hsiao C Y, Tzen C Y, Chang K T, et al.Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer[J].Clin Cancer Res, 2005, 11:3750-3757. |
| [18] | Han S W, Kim T Y, Hwang P G, Jeong S, Kim J, Choi I S, et al.Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib[J].J Clin Oncol, 2005, 23:2493-2501. |
| [19] | Pallis A G, Voutsina A, Kalikaki A, Souglakos J, Briasoulis E, Murray S, et al.'Classical' but not 'other' mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer[J].Br J Cancer, 2007, 97:1560-1566. |
| [20] | Lopez-Chavez A, Carter C A, Giaccone G.The role of KRAS mutations in resistance to EGFR inhibition in the treatment of cancer[J].Curt Opin Investig Drugs, 2009, 10:1305-1314. |
| [21] | Barbieri C E, Perez C A, Johnson K N, Ely K A, Billheimer D, Pietenpol J A.IGFBP-3 is a direct target of transcriptional regulation by DeltaNp63alpha in squamous epithelium[J].Cancer Res, 2005, 65:2314-2320. |
| [22] | Pelosi G, Pasini F, Olsen Stenholm C, Pastorino U, Maisonneuve P, Sonzogni A, et al.p63 immunoreactivity in lung cancer:yet another player in the development of squamous cell carcinomas?[J].J Pathol, 2002, 198:100-109. |
| [23] | Massion P P, Taflan P M, Jamshedur Rahman S M, Yildiz P, Shyr Y, Edgerton M E, et al.Significance of p63 amplification and overexpression in lung cancer development and prognosis[J].Cancer Res, 2003, 63:7113-7121. |
 2014, Vol. 35
2014, Vol. 35


