2. 兰州军区兰州总医院神经外科, 兰州 730050;
3. Department of Neurosurgery, College of Medicine, Mayo Clinic, 200 1st St SW, Rochester, MN 55905, USA
2. Department of Neurosurgery, Lanzhou General Hospital, PLA Lanzhou Military Area Command, Lanzhou 730050, Gansu, China;
3. Department of Neurosurgery, College of Medicine, Mayo Clinic, 200 1st St SW, Rochester, MN 55905, USA
颅内动脉瘤是常见的脑血管病,患病率为3.2%[1]。动脉瘤性蛛网膜下隙出血是目前已知的最具破坏性的神经系统疾病之一,发病1个月内病死率高达35%[2],幸存者中50%遗留重度残疾[3];而且蛛网膜下隙出血可导致严重的认知功能障碍,甚至恢复良好重返日常生活的患者中也常见认知功能障碍[4]。动脉瘤的发病机制目前尚不明确,无法进行有效防治。采用人类动脉瘤标本研究存在诸多限制,因此有必要探讨合适的颅内动脉瘤动物模型。大鼠、小鼠颅内动脉瘤模型,因脑血管过于细小,无法进行血管内治疗研究[5,6,7],而灵长类如猴子动脉瘤模型因伦理、费用等问题,难以广泛应用[8]。因此,本研究选择新西兰大白兔作为实验动物,采用双侧颈总动脉结扎,改变基底动脉顶端血流动力学,尝试构建更符合人类动脉瘤发病特点的动脉瘤动物模型。
1 材料和方法 1.1 实验动物及主要材料新西兰大白兔26只,由第二军医大学实验动物中心提供[动物许可证编号:SCXK(沪)2012-0007]。 将西兰大白兔随机分为实验组(n=20)和假手术组(n=6), 雌雄不限,体质量2.4~3.0 kg,平均(2.6±0.2) kg。饲养条件:室温(22±4)℃,相对湿度(58±6)%,光照与黑暗循环时间为12 h,饮水不限。 麻醉药物速眠新Ⅱ(吉林省华牧动物保健品有限公司,批号: 30106),戊巴比妥钠(上海西唐生物科技有限公司,德国进口分装,批号:20080518)。
1.2 动脉模型的建立及观察 1.2.1 麻醉及双侧颈总动脉结扎肌注速眠新Ⅱ 0.2 mL,动物安静后耳缘静脉注射2.5%戊巴比妥钠30 mg/kg。麻醉成功后,动物仰卧于手术台,颈部剃毛。常规碘伏、乙醇消毒、铺巾。取颈正中切口,长约1.5 cm,切开皮肤、皮下,钝性游离双侧颈总动脉,注意保护迷走神经,3-0丝线结扎双侧颈总动脉,缝合皮下组织及皮肤。假手术组仅游离双侧颈总动脉,不行结扎。术后正常饲养。 1.2.2 经颅多普勒超声(TCD)及脑血管造影检查
分别于结扎前(0 d)、术后1 d、1周、4周以TCD测量基底动脉血流速度。双侧颈总动脉结扎4周后行脑血管造影。全麻后,将新西兰大白兔固定于检查板上,右侧股部切开,暴露股动脉,远端结扎,滴2滴2%利多卡因减轻血管痉挛。穿刺针直接穿刺股动脉,顺利置入导丝后,拔出穿刺针,沿导丝置入5F动脉鞘。5F造影管超选左侧椎动脉造影。 1.2.3 血管组织的获取及观察
分别于术后1周、4周将动物处以安乐死,立即用肝素化生理盐水、10% 甲醛磷酸盐缓冲液维持压力在150 mmHg(1 mmHg=0.133 kPa),经心脏灌注20 min。仔细取出脑组织,固定于10%甲醛磷酸盐缓冲液至少24 h。游离基底动脉及分叉部。石蜡包埋,以4 μm厚度连续纵向切片,分别行EVG染色和Masson染色。 1.3 统计学处理
采用SPSS 17.0统计学软件进行分析,计量资料以 ±s表示,组间、不同时间点血流速度比较采用重复测量方差分析。检验水准(α)为0.05。
2 结 果 2.1 手术成功率假手术组动物全部成活,实验组4只兔死亡,其中1只麻醉后未清醒,死亡;1只术后1 d死亡;1只术后持续精神差,进食少,1周后明显消瘦,全身衰竭死亡;1只术后3周持续腹泻,死亡;存活16只,双侧颈总动脉结扎术后存活率为80%(16/20)。 2.2 基底动脉血流速度
双侧颈总动脉结扎后1 d,基底动脉血流速度直线升高,高于术前226%,差异有统计学意义(P<0.01)。术后1周仍在持续升高,术后4周血流速度较前下降,处于稳定状态。方差分析表明, 两组之间血流速度差异有统计学意义(P<0.01);实验组术后1周及4周之间差异无统计学意义(P=0.697)。实验组术后基底动脉血流速度较术前明显增加,假手术组血流速度变化不明显。具体见表 1。
| 表 1 假手术组及实验组兔基底动脉血流速度的变化Tab 1 Flow velocity of rabbit basilar artery in the sham and experimental groups ±s,qV/(mL·min-1) |
脑血管造影及血管标本大体观察结果表明: 假手术组血管形态无明显变化(图 1A、图 2A);实验组双侧颈总动脉结扎术后1周,基底动脉迂曲,迂曲方向延续主侧椎动脉走向,到达基底动脉中部再向相反方向扭曲(图 1B);术后4周,实验组基底动脉管腔直径较假手术组明显增大;形态进一步迂曲扩张(图 1C、图 2B)。
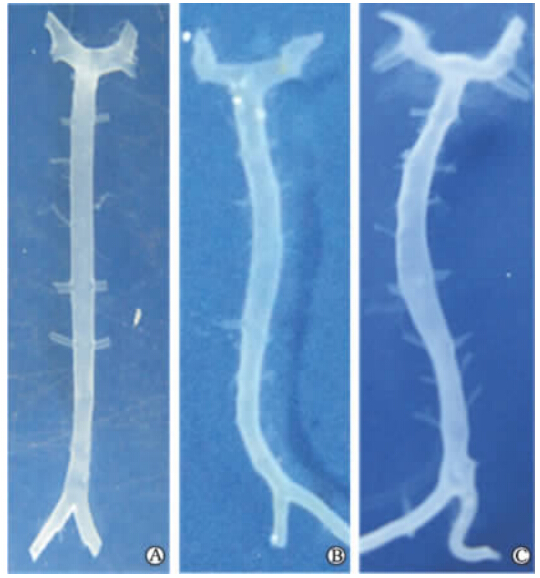 | 图 1 假手术组和实验组兔基底动脉形态变化Fig 1 Morphological changes of rabbit basilar artery in the sham and experimental groupsA: One week after the operation in the sham group; B: One week after the operation in the experimental group; C: Four weeks after the operation in the experimental group |
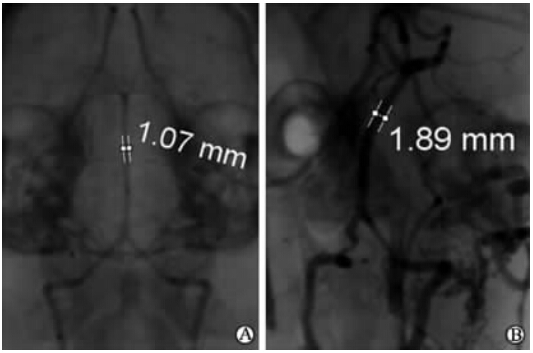 | 图 2 假手术组和实验组血管造影Fig 2 DSA findings in the sham and experimental groupsA: Four weeks after the operation in the sham group; B: Four weeks after the operation in the experimental group. Data were the lumen diameter of the basilar artery |
假手术组EVG染色显示内弹力层保持连续、完整(图 3A);Masson染色显示中膜厚度保持一致(图 3B)。而实验组EVG染色显示内弹力层断裂,部分消失或变薄(图 3C);Masson染色显示中膜变薄(图 3D)。
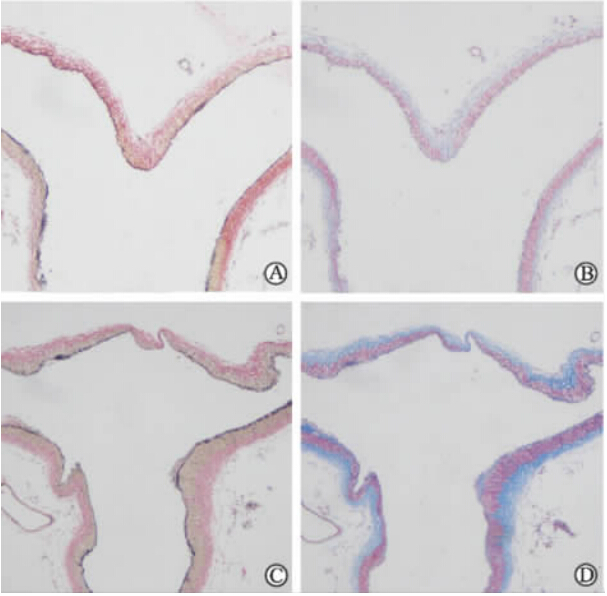 | 图 3 术后4周兔基底动脉顶端病理染色Fig 3 Pathological staining of rabbit basilar artery terminus 4 weeks after the operation A,C: EVG staining; B,D: Masson staining. A,B: Sham group; C,D: Experimental group. Original magnification: ×100 |
颅内动脉瘤因其高致死、致残率,严重威胁着人类的健康;但是其发病原因仍不明确,无法从根本上预防及治疗。为了探寻人类颅内动脉瘤的病因,研究者设计了不少动脉瘤模型,如静脉囊袋移植动脉瘤模型[9],利用弹性蛋白酶消化颈动脉残端动脉瘤模型[10,11]。但这些模型与人类动脉瘤生成没有因果关系,仅适合栓塞材料等方面的研究,不适合病因学研究。此外,还有利用血流动力学改变如结扎颈总动脉,结合诱导高血压和破坏弹力纤维(β-氨基丙腈)诱导大鼠、小鼠颅内动脉瘤模型[5,6,7],但这些小动物模型的脑血管过于细小,无法进行血流动力学研究及进一步血管内治疗研究。而灵长类如猴子动脉瘤动物模型因伦理问题、来源较少及费用昂贵等问题难以广泛应用[8]。兔颈总动脉直径与人类大脑中动脉起始段非常接近,同时凝血系统与人类最为接近;单纯改变血流动力学从病因学上更符合人类动脉瘤的发病特点。目前,约有33.6%的颅内动脉瘤实验采用兔制作模型[12]。
颅内动脉血管壁由完整的内弹力层、相对薄的中膜及外膜构成,这种结构特征一旦出现完整性破坏很容易形成动脉瘤[13]。血流动力学不稳定是早期颅内动脉瘤发生和破裂的重要因素[14]。基于以往文献[15,16],本研究选择新西兰大白兔作为实验动物,采取双侧颈总动脉结扎,改变基底动脉血流动力学,尤其增大基底动脉顶端壁面切应力,促进动脉瘤生成。这种动脉瘤模型制作相对简单,价格低廉,术后存活率较高,适合常规实验研究。结扎术后4周,造影检查见基底动脉明显增粗、迂曲,基底动脉顶端显微镜下见小囊泡样突出,病理切片EVG染色显示内弹力层断裂、部分消失,Masson染色示中膜明显变薄,符合人动脉瘤病理学特征。
通过新西兰大白兔双侧颈总动脉结扎,增加基底动脉顶端血流动力学,不附加其他任何危险因素,能够诱导出颅内动脉瘤。这与人颅内血管偶然闭塞或外科手术闭塞后其他血管血流增加,逐渐形成颅内动脉瘤[17,18]的机制一致。双侧颈总动脉结扎后,兔基底动脉血流速度明显增加,以弥补前循环缺血状态。而基底动脉血流速度增加寄望于Willis 环解剖结构畅通。Willis 环不通畅,前循环缺血状态无法改善,兔神经功能障碍表现明显,精神差,进食少,最终衰竭死亡。本实验有3只兔为此种原因死亡。基底动脉顶端高血流量导致这一区域壁面切应力及壁面切应力梯度进行性升高。局部高壁面切应力会诱导血管内皮细胞、血管平滑肌细胞等功能的变化,导致平滑肌细胞凋亡,胶原纤维和弹力纤维断裂和消失,从而内弹力层缺失,中膜变薄,导致血管张力丧失,为达到血流动力学的平衡,血管壁结构开始重塑形[19],血管延长、扩张、迂曲;且随时间延长,迂曲、扩张程度愈明显。当血流动力学的改变超过血管壁重建的限度时,就会导致局部动脉壁变薄和异常膨出,形成动脉瘤[20]。
兔越来越多地应用于动脉瘤模型的制作,但血流动力学改变诱导动脉瘤生成时间长,且主要为血管显微结构的改变,形成影像学可见的动脉瘤可能需要6个月以上时间,不利于短期治疗研究。
总之,双侧颈总动脉结扎所致血流动力学改变可诱导兔基底动脉顶端出现新生动脉瘤样改变,制作简单,费用相对低廉,动物存活率高,值得进一步研究以应用于动脉瘤研究。
4 利益冲突所有作者声明本文不涉及任何利益冲突。
| [1] | Vlak M H, Algra A, Brandenburg R, Rinkel G J.Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period:a systematic review and meta-analysis[J].Lancet Neurol, 2011, 10:626-636. |
| [2] | Stegmayr B, Eriksson M, Asplund K.Declining mortality from subarachnoid hemorrhage:changes in incidence and case fatality from 1985 through 2000[J].Stroke, 2004, 35:2059-2063. |
| [3] | Nieuwkamp D J, Setz L E, Algra A, Linn F H, de Rooij N K, Rinkel G J.Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region:a meta-analysis[J].Lancet Neurol, 2009, 8:635-642. |
| [4] | Wermer M J, Kool H, Albrecht K W, Rinkel G J;Aneurysm Screening after Treatment for Ruptured Aneurysms Study Group.Subarachnoid hemorrhage treated with clipping:long-term effects on employment, relationships, personality, and mood[J].Neurosurgery, 2007, 60:91-97. |
| [5] | Hashimoto N, Handa H, Hazama F.Experimentally induced cerebral aneurysms in rats[J].Surg Neurol, 1978, 10:3-8. |
| [6] | Morimoto M.Mouse model of cerebral aneurysm:experimental induction by renal hypertension and local hemodynamic changes[J].Stroke, 2002, 33:1911-1915. |
| [7] | Nuki Y, Tsou T L, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T.Elastase-induced intracranial aneurysms in hypertensive mice[J].Hypertension, 2009, 54:1337-1344. |
| [8] | Hashimoto N, Kim C, Kikuchi H, Kojima M, Kang Y, Hazama F.Experimental induction of cerebral aneurysms in monkeys[J].J Neurosurg, 1987, 67:903-905. |
| [9] | Spetzger U, Reul J, Weis J, Bertalanffy H, Thron A, Gilsbach J M.Microsurgically produced bifurcation aneurysms in a rabbit model for endovascular coil embolization[J].J Neurosurg, 1996, 85:488-495. |
| [10] | Cloft H J, Altes T A, Marx W F, Raible R J, Hudson S B, Helm G A, et al.Endovascular creation of an in vivo bifurcation aneurysm model in rabbits[J].Radiology, 1999, 213:223-228. |
| [11] | 王奎重, 刘建民, 黄清海, 洪 波, 许 奕, 赵文元.改良的胰弹性蛋白酶诱导兔囊状动脉瘤模型[J].中华神经外科杂志, 2010, 26:744-747. |
| [12] | Bouzeghrane F, Naggara O, Kallmes D F, Berenstein A, Raymond J;The International Consortium of Neuroendovascular Centres.In vivo experimental intracranial aneurysm models:a systematic review[J].AJNR Am J Neuroradiol, 2010, 31:418-423. |
| [13] | Schievink W I.Intracranial aneurysms[J].N Engl J Med, 1997, 336:28-40. |
| [14] | Baek H, Jayaraman M V, Richardson P D, Karniadakis G E.Flow instability and wall shear stress variation in intracranial aneurysms[J].J R Soc Interface, 2010, 7:967-988. |
| [15] | Gao L, Hoi Y, Swartz D D, Kolega J, Siddiqui A, Meng H.Nascent aneurysm formation at the basilar terminus induced by hemodynamics[J].Stroke, 2008, 39:2085-2090. |
| [16] | Metaxa E, Tremmel M, Natarajan S K, Xiang J, Paluch R A, Mandelbaum M, et al.Characterization of critical hemodynamics contributing to aneurysmal remodeling at the basilar terminus in a rabbit model[J].Stroke, 2010, 41:1774-1782. |
| [17] | Wolf R L, Imbesi S G, Galetta S L, Hurst R W, Sinson G P, Grossman R I.Development of a posterior cerebral artery aneurysm subsequent to occlusion of the contralateral internal carotid artery for giant cavernous aneurysm[J].Neuroradiology, 2002, 44:443-446. |
| [18] | Lee G Y, Brophy B P.Recurrent subarachnoid haemorrhage from a de novo basilar bifurcation aneurysm:a case report[J].J Clin Neurosci, 2003, 10:250-252. |
| [19] | Chatziprodromou I, Tricoli A, Poulikakos D, Ventikos Y.Haemodynamics and wall remodelling of a growing cerebral aneurysm:a computational model[J].J Biomech, 2007, 40:412-426. |
| [20] | Tateshima S, Tanishita K, Vinuela F.Hemodynamics and cerebrovascular disease[J].Surg Neurol, 2008, 70:447-453. |
 2014, Vol. 35
2014, Vol. 35


