原发性肝癌(hepatocellular carcinoma,HCC)是消化系统最常见的恶性肿瘤之一,其发病率和死亡率均居高不下,目前手术是治疗HCC最有效的方法,但术后复发率较高[1,2]。研究[3,4]发现肝癌的高复发率和高死亡率与肿瘤干细胞关系密切,肝癌干细胞可能在肝癌早期已进入外周血液,并且在外周血中无限增殖,诱导正常肝细胞和肿瘤细胞的恶变,此外肝癌干细胞对化疗药物和放疗的治疗效应也产生对抗作用,为降低肝癌的死亡率和复发率,早期发现肝癌患者外周血中的肝癌干细胞已成为目前研究的热点。CD133是肿瘤干细胞的特征性标记物之一[5,6],CD105是肿瘤微血管标记物之一且与CD133关系密切,两者共同参与了肿瘤的发生和发展[7]。本研究拟通过检测HCC患者外周血CD133和CD105阳性细胞的水平,探讨二者在肝癌发生、发展及预后判断中的重要意义。 1 资料和方法 1.1 研究对象
2003年3月至2009年3月在安徽医科大学第三附属医院进行手术治疗的HCC患者216例,本研究回顾性分析了其中在知情同意下行肝门区域淋巴结清扫+肝脏切除术的肝癌患者80例。该80例患者术前常规检查AFP、右上腹CT增强扫描,其中25例行上腹部MRI,未发现有远处转移。术后病理诊断为肝细胞肝癌63例(伴肝硬化54例),胆管细胞癌11例(合并肝内胆管结石5例、伴肝硬化3例),混合型肝癌6例均伴有肝硬化。80例HCC患者中术前21例发生淋巴结转移,病理组织示肝细胞癌13例、胆管细胞癌5例、混合性肝癌3例。术中无死亡。80例患者中男41例、女39例,平均年龄(58.22±7.00)岁,分别于术前5~7 d收集患者外周血进行CD133、CD105检测。选取正常健康体检者20例作为对照,其中男 13例、女7例,平均年龄(57.74±8.28) 岁。所有HCC患者均经病理学证实,术前未经过放化疗和免疫治疗,术后都经正规的放化疗,所有患者均无其他系统恶性肿瘤病史。 1.2 主要试剂与仪器
抗人CD105-FITC抗体、抗人CD133-PE抗体、抗人CD45-PCy5抗体、FITC、PE和PCy5标记的小鼠IgG1同型对照、FC-500流式细胞仪、溶血素、离心机均购自美国Beckman Coulter 公司。磷酸盐缓冲液(PBS)购自大冢(中国)投资有限公司。 1.3 实验方法
所有观察对象于清晨空腹抽取外周静脉血2 mL。EDTA抗凝,2 h内送至流式细胞仪室,取流式细胞仪专用试管2支,A管中加CD105-FITC抗体、抗人CD133-PE抗体、抗人CD45-PCy5抗体各10 μL,B管中加相应同型对照液各10 μL,A、B管分别加入100 μL全血,混匀后避光反应约15 min,各加溶血素1 mL,再避光放置10 min,以1 500 r/min的速度离心5 min,弃上清液,各加缓冲液2 mL,同一速度离心5 min,弃上清液,加400 μL缓冲液后上机,用流式细胞仪检测,以Cell Quest软件获取分析数据,定量分析每管样品中30 000个细胞,以CD45-/CD133+/CD105+细胞为阳性细胞,记录阳性细胞百分率,减去非特异性对照值。 1.4 统计学处理
采用SPSS16.0软件进行统计学分析,计量资料以x±s表示,组间比较采用t检验,相关性分析采用Pearson列联系数r计算,采用Kaplan-Meier方法进行生存率分析,应用Cox比例风险模型进行多因素分析。检验水准(α)为0.05。 2 结 果 2.1 术前、术后外周血CD45-/CD133+/CD105+细胞水平变化
80例HCC患者术前外周血CD45-/CD133+/CD105+细胞水平为(0.632±0.562)%,术后为(0.437±0.379)%,术前外周血中CD45-/CD133+/CD105+细胞水平高于术后(t=3.252,P=0.003);且较健康对照组[(0.025±0.071)%]升高,差异有统计学意义(t=4.801,P=0.000)。此外,HCC患者术后复发组术前外周血CD45-/CD133+/CD105+细胞水平[(0.770±0.608)%]高于术后未复发组[(0.465±0.389)%],差异有统计学意义(t=2.489,P=0.015)。 2.2 CD45-/CD133+/CD105+细胞表达与临床病理学特征的关系
患者外周血CD45-/CD133+/CD105+细胞比例与性别、年龄无关。临床分期Ⅱ~Ⅲ期肝癌患者外周血CD45-/CD133+/CD105+细胞比例高于Ⅰ~Ⅱ期肝癌患者,差异有统计学意义(t=2.734,P=0.008)。高、中、低分化癌患者外周血CD45-/CD133+/CD105+细胞比例,两两比较差异均有统计学意义(均P<0.05)。有淋巴结转移肝癌患者外周血CD45-/CD133+/CD105+细胞比例高于无淋巴结转移者,差异有统计学意义(t=3.110,P=0.003)。具体数据见表 1。
|
|
表 1 80例HCC患者CD45-/CD133+/CD105+细胞水平与临床特征的关系 |
80例HCC患者外周血中CD133随CD105阳性表达改变而改变者62例,二者呈正相关(r=0.846,P<0.05)。 2.4 CD45-CD133+、CD45-CD105+细胞表达水平与肝癌患者术后生存率的关系
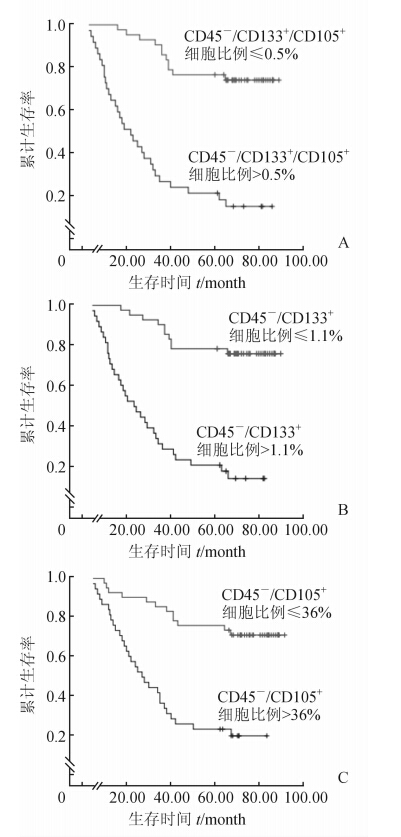
对80例HCC患者电话或信件随访并进行Kaplan-Meier分析,比较其外周血中CD45-/CD133+/CD105+细胞的不同表达水平与生存率的关系,总生存期定义为自手术之日起至随访截止或死亡。外周血CD45-/CD133+/CD105+细胞比例>0.5%者术后生存率较细胞比例≤0.5%者降低(P<0.05,图 1A),CD45-/CD133+细胞比例>1.1%者术后生存率较细胞比例≤1.1%者降低(P<0.05,图 1B),CD45-/CD105+细胞比例>36%者术后生存率较细胞比例≤36%者降低(P<0.05,图 1C)。
|
图 1 CD45-/CD133+/CD105+、CD45-/CD133+和CD45-/CD105+的细胞表达水平与肝癌患者术后生存率的关系 |
外周血中CD45-/CD133+/CD105+细胞表达水平、分化程度、有无淋巴结转移是影响本组肝癌患者预后的独立因素(表 2)。
|
|
表 2 影响原发性肝癌术后预后的Cox模型多因素分析 |
肝癌干细胞的研究尚处于初级阶段,研究发现在结肠癌、前列腺癌、脑胶质瘤中均存在CD133阳性细胞,并且与肿瘤的恶性程度密切相关[8,9,10]。CD105参与血管新生过程,从而促进肿瘤的生长、浸润和转移[11,12,13]。本课题组前期探讨了CD133和CD105阳性细胞在HCC患者中的增殖及侵袭作用,联合检测CD133和CD105双阳性细胞可以更加精确地筛选出肝癌干细胞,其部分实验结果已发表[14],此外,国外文献也已有相关实验[15,16]。
由于CD133+和CD105+的细胞在外周血中非常少,因此,需要利用CD45进行富集以提高检测灵敏度。抗人CD45抗体又称抗人白细胞共同抗原抗体,它可以结合血源性白细胞,而不结合非血源性的肿瘤细胞。在使用流式细胞仪对单个核细胞检测时,考虑到CD45阴性的单核细胞可以从分离液和血浆之间分离[17,18],我们首先加CD45 Per CP以进行CD45/SSC双参数散电图设门,选中CD45-区域再进行下一步分析,从而将外周血单个核细胞中血源性白细胞去除,使该CD45-区域细胞全为肿瘤细胞,在此基础上进一步检测CD133、CD105阳性表达,可使CD133+CD105+肝癌干细胞的检测敏感性大幅提高。
本研究结果显示,术后肝癌患者外周血CD45-/CD133+/CD105+细胞水平低于术前(P<0.05),而健康正常人外周血几乎未见CD45-/CD133+/CD105+细胞[19]。这表明手术切除肿瘤后,外周血CD45-/CD133+/CD105+细胞比例下降,这有助于延缓肿瘤的进一步发展。本研究还发现CD45-/CD133+/CD105+细胞的表达与临床分期、分化程度以及淋巴结转移相关,有文献报道术中切除局部淋巴结可能提高患者预后[1, 20]。因此推测肝癌患者外周血CD45-/CD133+/CD105+细胞水平越高,患者的病情越严重,预后越差,原因可能与含有的肿瘤干细胞越多,新生血管增生越活跃,转移几率进一步增加有关[21]。
本研究还发现,肝癌患者外周血中CD133与CD105的表达呈正相关,即肝癌外周血中CD133表达越高,CD105表达也越高。这可能是因为CD133作为一种肝癌干细胞,其可以促进或分化成血管内皮细胞,从而导致CD105的表达升高,促进肿瘤的生长、浸润和转移。
综上所述,肝癌患者外周血CD45-/CD133+/CD105+细胞表达水平明显升高,且与肝癌的恶性程度密切相关,另外其还极大地影响着患者预后,对CD45-/CD133+/CD105+细胞表达的进一步研究,可能会为预测肝癌复发及判断肝癌预后提供新的依据,靶向降低肝癌患者外周血CD45-/CD133+/CD105+细胞的数量将为肝癌的临床治疗提供一个应用前景。
4 利益冲突
所有作者声明本文不涉及任何利益冲突。
| [1] | Kim K H,Lee S G,Park E H,Hwang S,Ahn C S,Moon D B,et al.Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma[J].Ann Surg Oncol,2009,16:623-629. |
| [2] | 徐本玲, 袁 龙, 高全立, 范瑞华, 张成娟, 宋永平.重组纤维连接蛋白诱导自体CIK联合IFN-α治疗晚期肝癌疗效[J].郑州大学学报(医学版), 2011,46:694-696. |
| [3] | Kohga K,Tatsumi T,Takehara T,Tsunematsu H, Shimizu S,Yamamoto M,et al.Expression of CD133 confers malignant potential by regulating metalloproteinases in human hepatocellular carcinoma[J].J Hepatol,2010,52:872-879. |
| [4] | Piao L S,Hur W,Kim T K,Hong S W,Kim S W,Choi J E,et al.CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma[J].Cancer Lett,2012,315:129-137. |
| [5] | Pallini R,Ricci-Vitiani L,Montano N,Mollinari C,Biffoni M,Cenci T,et al.Expression of the stem cell marker CD133 in recurrent glioblastoma and its value for prognosis[J].Cancer,2011,117:162-174. |
| [6] | Song W,Li H,Tao K,Li R,Song Z,Zhao Q,et al.Expression and clinical significance of the stem cell marker CD133 in hepatocellular carcinoma[J].Int J Clin Pract,2008,62:1212-1218. |
| [7] | 程继荣,王淑琴,祖木热提, 邹 静.肺癌组织中CD133和CD105的表达及其临床意义[J].肿瘤, 2010,30:334-337. |
| [8] | Park B S,Jung S Y,Kwon S M,Bae J H,Lee S M,Shin D H,et al.Comparison of putative circulating cancer stem cell detection between the hepatic portal system and peripheral blood in colorectal cancer patients[J].Ann Surg Treat Res,2014,87:232-238. |
| [9] | Miki J,Furusato B,Li H,Gu Y,Takahashi H,Egawa S,et al.Identification of putative stem cell markers,CD133 and CXCR4,in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens[J].Cancer Res,2007,67:3153-3161. |
| [10] | Singh S K,Hawkins C,Clarke I D,Squire J A,Bayani J,Hide T,et al.Identification of human brain tumour initiating cells[J].Nature,2004,432:396-401. |
| [11] | Li Y,Zhai Z,Liu D,Zhong X,Meng X,Yang Q,et al.CD105 promotes hepatocarcinoma cell invasion and metastasis through VEGF[J].Tumour Biol,2014 Oct 7.[Epub ahead of print] |
| [12] | Miyata Y,Mitsunari K,Asai A,Takehara K,Mochizuki Y,Sakai H.Pathological significance and prognostic role of microvessel density,evaluated using CD31,CD34,and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy[J].Prostate,2014 Oct 13.[Epub ahead of print] |
| [13] | Yoshitomi H,Kobayashi S,Ohtsuka M,Kimura F, Shimizu H,Yoshidome H,et al.Specific expression of endoglin (CD105) in endothelial cells of intratumoral blood and lymphatic vessels in pancreatic cancer[J].Pancreas,2008,37:275-281. |
| [14] | 张俊松, 汪 宏, 吴立胜.CD105、CD133在人肝癌细胞株HepG-2的表达情况及生物学性状的体外研究[J].肝胆外科杂志, 2013, 21:133-136. |
| [15] | Lee J S,Heo J,Libbrecht L,Chu I S,Kaposi-Novak P,Calvisi D F,et al.A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells[J].Nat Med,2006,12:410-416. |
| [16] | Tang Y,Kitisin K,Jogunoori W,Li C,Deng C X,Mueller S C,et al.Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling[J].Proc Natl Acad Sci USA,2008,105:2445-2450. |
| [17] | Jacob K,Sollier C,Jabado N.Circulating tumor cells: detection,molecular profiling and future prospects[J].Expert Rev Proteomics,2007,4:741-756. |
| [18] | Naume B,Borgen E,Tssvik S,Pavlak N,Oates D,Nesland J M.Detection of isolated tumor cells in peripheral blood and in BM: evaluation of a new enrichment method[J].Cytotherapy,2004,6:244-252. |
| [19] | Ma S.Biology and clinical implications of CD133(+) liver cancer stem cells[J].Exp Cell Res,2013,319:126-132. |
| [20] | Agrawal S,Belghiti J.Oncologic resection for malignant tumors of the liver[J].Ann Surg,2011,253:656-665. |
| [21] | Hou Y,Zou Q,Ge R,Shen F,Wang Y.The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers[J].Cell Res,2012,22:259-272. |
 2014, Vol. 35
2014, Vol. 35


