2. 三峡大学第一临床医学院妇产科, 宜昌 443002;
3. 三峡大学第二临床医学院妇产科, 宜昌 443002
2. Department of Obstetrics and Gynecology, First College of Clinical Medical Science, China Three Gorges University, Yichang 443002, Hubei, China;
3. Department of Obstetrics and Gynecology, Second College of Clinical Medical Science, China Three Gorges University, Yichang 443002, Hubei, China
人乳头瘤病毒(human papillomavirus,HPV)主要外壳蛋白(major capsid protein,L1),具有良好的抗原性,可以诱导机体产生强烈的体液和细胞免疫反应[1, 2]。弄清肿瘤组织、细胞内HPV L1表达的规律,对了解HPV所致肿瘤的肿瘤生物学、肿瘤靶向治疗和HPV L1治疗性疫苗的研究都有重要的意义。
既往的观点认为,HPV病毒一旦导致肿瘤形成,肿瘤组织内是不再会有L1蛋白表达的。这一观念的建立,主要基于对HPV生物学的两点认识。首先,HPV L1蛋白表达与鳞状上皮表层角质形成细胞的成熟分化过程有关,只有在鳞状上皮表层细胞开始进入分化状态,启动角质形成过程,HPV病毒的生活周期才能完成,才能有L1蛋白的表达[3, 4]。在肿瘤组织和高级别病变中,由于肿瘤组织的去分化,病毒不能启动晚期蛋白的表达程序,因而,也就没有L1蛋白表达[3, 4]。其次,由于病毒DNA基因组整合入宿主DNA中,病毒基因组发生了重排,使原来处于HPV L1基因上游的启动序列,被转移到了L1基因的下游,L1基因的转录不能启动,没有L1 mRNA的产生,L1蛋白就不会表达[5]。但是,既往的这一观点与我们研究观察到的现象[6]和一些研究者的报道[7, 8]有所不同。为此,我们对HPV病毒基因组插入宿主基因的状况进行分析,认为在HPV所致病变及肿瘤组织内L1应该有表达的基础,为了验证上述推断是否正确,本研究通过对HPV L1在不同宫颈病变组织中的表达现象加以归纳并进行理论分析,以期在证明肿瘤组织内有L1表达的现象时,给予相应的理论解释。 1 理论基础 1.1 HPV L1可能在肿瘤组织表达
首先,如果肿瘤组织内含有游离HPV病毒基因组(病毒基因组的物理状态为混合态,同时含有整合态基因组和游离态基因组),而肿瘤组织的分化程度又比较高,HPV病毒生活周期的晚期事件就有可能随肿瘤细胞的分化被启动,使L1得到表达。
其次,L1蛋白的表达可以借助插入区段的宿主基因完成。病毒基因组为游离状态时,转录形成的病毒mRNA有两种类型——早期mRNA和晚期mRNA。但无论是早期mRNA,还是晚期mRNA,转录的起始序列都位于基因组的起始端,在E1基因之前;终止序列和多聚腺苷酸信号(polyadenylation signal,pA)都在基因组的后半段,E2之后[5]。病毒基因组整合入宿主基因序列时,病毒的环状双股脱氧核苷酸链从中部E2基因区段处被打开,病毒的核苷酸链状态由环形变成了线形,在有1 000~4 000碱基对丢失[9, 10, 11]的同时,病毒基因组的基因前后排列顺序同时发生了变化,即基因顺序发生了重排。此时,早期基因(E6、E7)的转录虽有起始序列,却没有终止序列和多聚腺苷酸信号可用;转录后的剪切虽然有5′给予剪切位点(splice donor site)可用,却没有3′接受剪切位点(splice acceptor site)可用。与上述情况相反的是,晚期基因(L1、L2)的转录虽有终止序列和多聚腺苷酸信号可用,却没有起始序列可用;转录后的剪切虽然有3′接受剪切位点可用,却没有5′给予剪切位点可用。对整合后病毒基因组基因排列的情况进行分析,我们可以得出这样的结论:HPV病毒的E6、E7和L1、L2基因如若表达,都必需利用宿主基因提供相应的序列和信号才能完成(图 1),也就是病毒基因组必须要插入宿主基因组的可编码序列,否则就是无效插入。Wentzensen 等[11]对来自于整合HPV16和18基因组的肿瘤组织和细胞内的E6/E7癌基因转录物进行分析,结果发现100%为病毒-细胞融合转录物。同理,根据上述分析和他人的研究[11, 12],我们基本上可以得出这样一个推断:如果不考虑移码阅读的情况,整合了HPV病毒基因组的宿主组织内,只要有E6、E7的表达,就存在L1,甚至L2的表达可能。由于晚期基因组的表达是借助于宿主的可编码基因,因此晚期基因的表达量就与插入点宿主基因的自身表达特性密切相关,或高或低,或随细胞生活状态的变化而改变。但有移码阅读的错误存在时,L1将无法表达(图 1)。
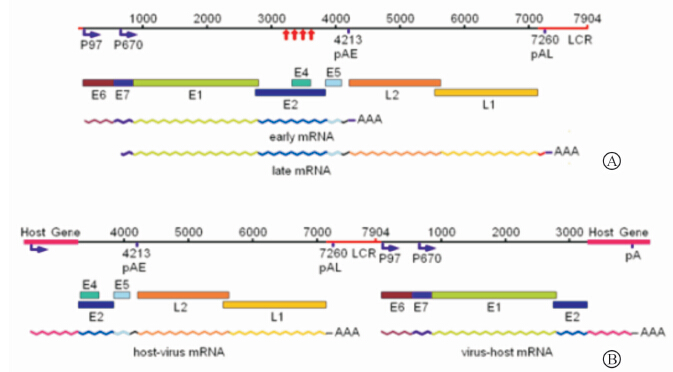 | 图 1 HPV16病毒基因组整合前后基因排列及基因转录物的变化分析图(根据参考文献[5, 11]改绘)Fig 1 Gene sequence and analysis of genetic transcript changes before and after HPV16 virus genome integration (redrawn according to references [5, 11]) A: The types of virus mRNA formed in transcription and positions of regulatory sites in viral genome related to the transcription when the viral genome was at episomal. The red arrows show that the sequences of genome was broken and lost during the integration of virus genome. B: The types of virus mRNA formed in transcription and positions of regulatory sites in viral genome related to the transcription after virus genome was integrated to the gene frame of the host. The loss of virus gene was not taken into consideration in this figure. |
由于病毒基因组在病变组织内物理状态的不同(游离、混合、整合)、肿瘤组织分化程度不同(分化、未分化或去分化或低分化)、病毒基因组整合后有无移码阅读的错误(正确转录、不转录或转录异常),HPV L1蛋白在肿瘤组织内的表达就会因上述条件的不同组合而有所不同(图 2)。
 | 图 2 细胞分化状态与HPV病毒基因组物理状态的不同组合对L1表达影响的分析图Fig 2 Analysis of L1 expression affected by different combinations of cell differentiation states and HPV virus genome physical states There are 10 combinations for HPV L1 protein expression in the tissue. Ⅰ: There are only episomal viruses;Ⅱ: There are episomal and integrated viruses. Ⅲ: There are only integrated viruses. α: Viral genome at episomal; β: Viral genome integrated into the sequence could be transcribed and read correctly;γ: Viral genome integrated into the sequence could not be transcribed or had frame shift reading. A: Undifferentiated,or dedifferentiated,or poorly differentiated;B: Differentiated or well differentiated |
根据组合情况的不同,至少可以得到10种组合形式,其中有6种组合是可以有L1蛋白的表达。(1)病毒基因组在游离状态下,L1的表达与细胞的分化有关,即当有角质蛋白合成启动时,也同时启动了L1蛋白的表达(图 2,Ⅰ-α-B)。(2)当病毒基因组发生了整合,如果没有移码阅读的错误存在,L1的表达将有两种情况:①在病毒基因组的物理状态为混合态时,L1的表达不仅与病毒基因组所插入的宿主基因的表达特性相关,还与分化相关(图 2,Ⅱ-β-A和B);②在病毒基因组的物理状态为纯整合态时,L1的表达就只与病毒基因组所插入的宿主基因的表达特性直接相关,而与分化无关(图 2,Ⅲ-β-A和B)。(3)当病毒基因组发生了整合,而有移码阅读的错误存在时,L1的表达将也有两种情况:①在病毒基因组的物理状态为混合态时,L1的表达受游离态的病毒基因组控制,与分化相关(图 2,Ⅱ-γ-A和B);②在病毒基因组的物理状态为纯整合态时,L1任何时候都无法表达,与分化无关(图 2,Ⅲ-γ-A和B)。 2 材料和方法 2.1 临床标本
新鲜宫颈组织标本均取自2008年3月至2010年9月宜昌市中心人民医院妇产科门诊及手术室治疗的患者,病变包括尖锐湿疣、慢性宫颈炎、宫颈上皮内瘤变(CIN)、原位癌、宫颈鳞状细胞癌和腺癌。取材前患者均未进行放疗及化疗处理。所有完成病理诊断的剩余标本,分作3份,分别用于DNA提取、蛋白提取和石蜡切片。 2.2 细胞培养
宫颈癌细胞株CaSki、SiHa、HeLa和HaCat均购自武汉大学中国典型培养物保藏中心。CaSki细胞为HPV16型阳性宫颈鳞状癌细胞系,HPV病毒基因组为游离状态绝对占优的混合型[12];SiHa细胞为HPV16型阳性宫颈鳞状癌细胞系,HPV病毒基因组为整合型[12];HeLa细胞为HPV18型宫颈腺癌细胞系,HPV病毒基因组为整合型[13]。HaCat细胞为人类永生化角质形成细胞系,HPV病毒基因组阴性。各细胞均用含10%小牛或胎牛血清的DMEM培养液在37℃、5%CO2、饱和湿度条件下进行培养。收获的细胞分别用于DNA提取、蛋白提取和免疫细胞化学检测。 2.3 引物设计
参照文献[10]设计引物,由上海生工生物工程有限公司合成。引物序列如下:HPV16 E6:5′-AAG GCG TAA CCG AAA TCG G-3′,5′-CAT ATA CCT CAC GTC GCA G-3′,产物长度206 bp;HPV16 E2:5′-ATG AAA ATG ATA GTA CAG AC-3′,5′-CCA GTA GAC ACT GTA ATA GA-3′,产物长度1 026 bp;HPV18 E6:5′-AAG GCA TAA CCG AAA TAG G-3′,5′-TTC TGC TGG ATT CAA CGG T-3′,产物长度452 bp;HPV18 E2:5′-ATG AAA ATG ACA GTA AAG AC-3′,5′-CAT TGT CAT GTA TCC CAC C-3′,产物长度1 028 bp。 2.4 试剂
小鼠抗HPV 1、6、11、16、18、31 L1多价单克隆抗体(货号MAB837)和小鼠抗HPV16 L1单克隆抗体CamVir-1(货号CBL402)为Millipore Corporation产品,兔HPV L1多克隆纯化抗体[14]由杭州华安生物技术有限公司制备。Ф10 nm胶体金标记羊抗小鼠IgG抗体为Sigma产品(货号G7652)。生物素-抗生物素-辣根过氧化物酶复合物及生物素化羊抗小鼠IgG抗体均为武汉谷歌生物科技有限公司产品。小鼠抗体免疫组化试剂盒为欣博盛生物科技有限公司产品。蛋白Marker为Fermentas产品(货号SM0672)。Sf9昆虫细胞-杆状病毒表达系统表达的HPV16和18 L1重组蛋白为中国医学科学院基础所许雪梅教授馈赠[15]。 2.5 临床标本及培养细胞DNA和蛋白的提取
(1)宫颈标本:收集后尽快冻存于-80℃冰箱备用,临用时取黄豆大小组织块,尽量剪碎后分装不同的1.5 mL EP管。(2)培养细胞:当细胞铺满培养瓶80%时,胰蛋白酶消化细胞,800 r/min离心后分装不同的1.5 mL EP管。(3)DNA的提取:于上述各EP管中分别加入10 mmol/L pH=8.0的TE缓冲液400 μL,20% SDS 25 μL,1 mg/mL蛋白酶K 50 μL,于55℃水浴箱中保温过夜。DNA按照标准酚氯仿抽提法提取。待絮状物出现后离心,向沉淀中加入50~100 μL双蒸水溶解过夜,分装于EP管中-20℃保存备用。(4)蛋白的提取:上述各EP管用常规单去污裂解液法提取蛋白,提取好的蛋白分装于EP管中,于-20℃保存备用。 2.6 HPV型别鉴定
采用PCR技术扩增HPV16和HPV18 E6基因片段。反应体系:2×Taq Master Mix 12.5 μL,DNA模板1.5 μL,HPV16和HPV 18 E6上、下游引物0.8 μL,双蒸水补足体积至25 μL。扩增条件:94℃预变性5 min;94℃变性1.5 min、55℃退火1 min、72℃延伸1.5 min,扩增30个循环;最后72℃充分延伸5 min。反应产物于1.5%的琼脂糖凝胶电泳,电压60~80 V,时间50 min;电泳后于凝胶成像系统照相保存。 2.7 HPV基因组物理状态的鉴定
对已确定HPV16、18型别的临床标本分别扩增其E2全长基因及E6基因片段。反应体系包括2×Taq Master Mix 12.5 μL,10 μmol/L HPVE6、E2引物各0.8 μL,DNA模板约1.5 μL,加双蒸水至总体积25 μL,混匀后稍离心,上机扩增。反应条件:由于E2产物跨越E2 ORF全长,长度大于1 kb,为使模板充分变性,总DNA首先于沸水中煮沸10 min。循环参数:94℃ 2 min、55℃ 2 min、72℃ 2 min,共35个循环。最后72℃充分延伸10 min。产物于1.5%的琼脂糖凝胶电泳,60~80 V电泳50 min后,凝胶成像系统照相。 2.8 HPV L1蛋白表达的ELISA检测
提取HPV16/18阳性宫颈标本组织蛋白,用HPV L1兔多肽抗体进行ELISA方法的HPV L1蛋白表达的检测。以永生化角质形成细胞(HaCat)为阴性对照。对所得光密度(D)值进行数值处理,采用公式(D待测-D空白)/(D阴性-D空白)进行计算,当所得值≥2.1时判定为阳性反应。 2.9 HPV L1蛋白表达的蛋白质印迹检测
将已经处理好的蛋白样品加入制备好的SDS-PAGE凝胶的上样孔中电泳,将电泳好的凝胶进行蛋白转膜。取出转膜后的NC膜,放入含3% BSA的封闭液中封闭;然后加小鼠多价抗HPV L1单克隆抗体反应;孵育后的膜洗后,再用1∶4 000的HRP标记的羊抗小鼠IgG抗体作用;用TBS洗,DAB显色液显色。 2.10 HPV L1蛋白表达的免疫组织化学检测
将石蜡切片标本脱蜡后,PBS洗,加入3%H2O2甲醛溶液去除内源性过氧化物酶;PBS洗,加入抗HPV L1抗体,孵育1 h;PBS洗,加入生物素化的羊抗鼠二抗;PBS洗,加入生物素-抗生物素-辣根过氧化物酶复合物;PBS洗,DAB显色;PBS洗,苏木精复染,水冲洗;脱水、透明、树胶封片、镜检。所有免疫组织化学检测的过程均以HPV6阳性的尖锐湿疣为阳性对照。抗体检测根据需要分别使用小鼠抗HPV1、6、11、16、18、31 L1多价单克隆抗体,小鼠抗HPV16 L1单克隆抗体CamVir-1,兔抗HPV L1多克隆抗体。 2.11 统计学处理
用SPSS10.0软件处理数据,结果以x±s表示,组间差异的比较采用单因素方差分析,检验水准(α)为0.05。 3 结 果 3.1 标本收集
总共收集到检测用临床标本48例,其中慢性宫颈炎22例,CIN Ⅰ~Ⅱ7例,原位癌4例,早期浸润癌7例,中晚期癌8例。另有尖锐湿疣3例(HPV6型阳性),用于免疫组织化学阳性对照。 3.2 HPV型别检测
标本通过PCR扩增,扩增产物经1.5%琼脂糖凝胶电泳,凝胶成像系统观察结果。采用HPV16、18两对特异性E6引物,对应扩增产物大小分别为397、322 bp(图 3)。主要筛选出HPV16、18两种主要HPV型别。
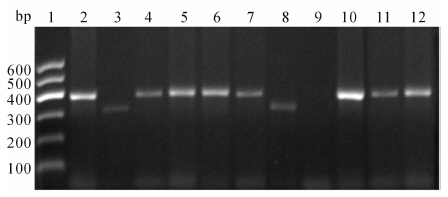 | 图 3 HPV型别检测E6引物PCR扩增产物电泳图Fig 3 Electrophoretogram of PCR amplification products with E6 primer for HPV type detection 1: DNA marker; 2: CaSki cells were used as positive control of HPV16 (397 bp); 3: HeLa cells were used as positive control of HPV18 (322 bp); 4-7,10-12: The specimens were HPV16 positive (397 bp); 8: The specimen was HPV18 positive(322 bp); 9: The specimen was of some other type,showing no band |
从所收集的48例宫颈病变组织中,我们筛选出42例HPV16感染(87.5%),3例HPV18感染(6.25%),非16、18型别共3例(6.25%)。见表 1。
|
|
表 1 宫颈标本组织HPV型别检测结果 Tab 1 Detection of HPV types in cervical tissue specimens |
对已确定HPV16、18型别的临床标本分别扩增其E2全长基因及E6基因片段,HPV16标本分别在206、1 026 bp处呈现条带,HPV18分别在452 bp、1 028 bp处出现条带。扩增后的PCR产物电泳条带用Scion Image4.0图像分析软件进行灰度分析,计算E2、E6条带面积灰度值,判断HPV基因组的物理状态[10]:(1)E2/E6≥0.8,为游离状态,泳道上有两条带,亮度基本相当;(2)0
汇总病毒基因组物理状态的检测结果,可以得出,随着病变程度的提高,游离型病毒基因组的比例越来越低,而整合型病毒基因组的比例越来越高。不同病变之间,病毒基因组的物理状态比较差异有统计学意义(表 2)。
ELISA检测结果显示,随着病变程度的加重,HPV L1的表达呈现逐渐下降的趋势。不同病变类型之间,HPV L1表达的比较差异具有统计学意义(图 5)。
检测结果显示,含有游离HPV16病毒基因组的宫颈炎、HPV基因均为混合型的CINⅠ~Ⅱ、原位癌及早期鳞状细胞癌组织,在55 000处均出现特异性反应条带。宫颈炎和CINⅠ~Ⅱ组织的反应条带明显,原位癌及早期鳞状细胞癌组织的反应条带明显变弱,显示反应强度有随组织病变程度的加重而逐渐变弱的趋势。中晚期鳞状细胞癌(HPV基因整合型)泳道上未见明显的反应条带出现(图 6)。
作为阳性对照的HPV6阳性的尖锐湿疣免疫组织化学检测显示,阳性反应均出现在上皮的中上层,并且主要集中于细胞核的位置(图 7A)。与对照不同,所有HPV16阳性及HPV18阳性的各类病变组织,阳性反应在细胞内的分布却主要出现于细胞核以外的区域,细胞核常呈负染状态。此外,阳性反应区域在整块组织内的分布及反应强度可以有很大的差别。
HPV L1阳性反应在不同的病变组织内可以有多种不同的分布特征,即便是同一类病变,是否有阳性反应,阳性反应的分布也有所不同。肿瘤组织内阳性反应的分布特征与组织内HPV基因组的物理状态(游离、混合、整合)、组织的分化程度(高分化、低分化)和肿瘤的进程(早期、晚期)等相关。反应规律大致可以分为两类:第一类,含有游离病毒基因组的病变或肿瘤组织,阳性反应强度与组织局部的分化程度呈正相关,按图 2细胞状态和病毒物理状态的组合划分,应归于Ⅰ-α-A和B(图 7A、7B)和Ⅱ-β-A和B(图 7E);如组织分化程度较低,或细胞分化状态比较一致,则组织内的反应强度呈均一状态,组合划分属于Ⅱ-β-A(图 7C)。第二类,所含病毒基因组为完全整合状态的肿瘤组织,阳性反应强度与组织局部的分化程度没有相关性,或强,或弱,或完全没有阳性反应,全组织反应强度相对一致,按图 2细胞状态和病毒物理状态的组合划分,应该包括有阳性反应的Ⅲ-β-A和B(图 7D、7G、7H),无阳性反应的Ⅲ-γ-A和B(图 7F,7I)。
从阳性反应趋势看,在早期病变组织中,经常可以看到有很强的阳性反应结果,但是在晚期病变中,看到的几乎都是弱阳性,或阴性结果,除了某些病变局部可以有强的阳性反应外,完全看不到全组织的强阳性反应(图 7E~7I)。病理类型相同的肿瘤,处于病程早期的肿瘤组织的阳性反应强度一般高于处于病程晚期的肿瘤组织。
4 讨 论
探讨HPV所致肿瘤内是否有L1蛋白的表达,主要目的是探索能否将L1蛋白作为HPV所致肿瘤的靶蛋白,以用于肿瘤生物治疗;探索能否将HPV感染预防性疫苗——HPV L1 VLP拓展成为HPV所致肿瘤的治疗性疫苗。
由HPV感染所致的肿瘤,发生瘤变之后的组织细胞之中是否有L1的表达,除了与肿瘤组织的分化状态有关,更与HPV病毒基因组整合至宿主基因组的位点有关[7, 11, 16]。有研究发现,病毒基因组在整合之后,有与宿主基因相关的融合蛋白表达。尽管HPV病毒基因组在整合入宿主基因时,会发生部分基因的丢失,但到目前为止的各类相关研究显示,涉及L1基因的丢失极少,基本保留完整[13, 17, 18]。以HeLa细胞为例,HeLa细胞内含两个整合HPV18病毒基因组,长度分别为7.8和6.9 kb,均带有完整的L1基因[13]。由于保留的HPV L1基因其起始部有3′剪切位点,后有晚期多聚腺苷酸序列信号[11, 19, 20],因此当HPV18基因组插入的位点恰是宿主基因组的可转录序列时,细胞的内环境如果合适,HPV18 L1基因就有可能被转录,转录物经恰当的剪切,L1蛋白最终就会得到表达[7, 21]。在对发生HPV整合的肿瘤组织作HPV基因转录物分析时,研究者发现有源于宿主染色体的HPV L1转录物存在[11, 12]。
既往的关于L1蛋白在瘤变组织内不表达的观点形成,除了有理论上的认识 [3, 4]外,还有一个重要支持,就是事实上在肿瘤组织内确实少有L1的检出,尤其是在晚期肿瘤中,极少有L1蛋白的检出。我们认为,大多数HPV所致肿瘤组织内不表达或低表达L1,除了因移码阅读错误所致不表达占有很大比例外(约2/3),在肿瘤发展的过程中,免疫系统的选择是一个非常重要的因素。肿瘤在不断异化的生长过程中,由于L1的强抗原性,所有高表达L1蛋白的细胞克隆都将被免疫系统选择掉,留下的都是L1低表达、不表达、或条件(分化)表达的肿瘤细胞克隆。既往研究证明,在HPV所致肿瘤患者的体内,可以检测到很强的针对L1蛋白的体液和细胞免疫反应[22, 23, 24]。所以我们看到的大多数的实体瘤都是L1蛋白低表达、不表达或中心(内部)表达的状况。这也就是为什么关于HPV L1在肿瘤组织内表达,包括许多研究都发现了这样一个现象,即随着肿瘤病变程度的加重,L1蛋白的表达会呈现逐渐下降的趋势[6, 7, 8]。本研究用ELISA方检测肿瘤组织内L1蛋白表达的结果也再次证实了这一现象。
ELISA检测结果显示,随着病变程度的加重,HPV L1的表达呈现逐渐下降的趋势。不同病变类型之间,HPV L1表达的比较差异具有统计学意义。结果说明在各类病变组织内虽然可以检测到有L1蛋白,但表达量是随着病变程度的加重而逐渐减少的。
蛋白质印迹检测结果显示,宫颈炎、CINⅠ~Ⅱ、原位癌、早期鳞状细胞癌组织均在55 000处出现特异性反应条带,但反应强度有逐渐变弱的趋势,说明上述这些组织内均有L1蛋白表达,但表达量随着病变程度的加重而逐渐减少。在中晚期鳞状细胞癌泳道上未见明显的反应条带出现,说明中晚期癌组织内没有HPV L1蛋白表达,或表达量太低而无法检测到。
本研究的免疫组织化学显示,L1阳性反应在肿瘤组织内的分布和反应强度具有多种表现形式,但是,我们均可以在一定程度上按图 2显示的细胞分化状态与HPV病毒状态的组合对这些反应现象进行分类,确定它们呈现这些反应现象所依托的细胞生物学和病毒生物学基础——细胞分化状态、病毒基因组物理状态和整合病毒基因组阅读状态。正是因为HPV致肿瘤细胞可以有多种不同的存在状态,所以才造成了差异很大、表现形式多样的免疫组化反应表现。通过对这些反应现象的合理解释,可以很好地解除许多研究者在得到差异很大的检测结果时产生的困惑,以致怀疑所得到的结果是否真实。
本研究的结果可能与本研究领域内既往形成的主流观点截然不同,但是根据来自多方面的研究报道及我们所做的理论分析,我们认为,宫颈癌组织或者细胞内有HPV L1表达应该是一种正常现象,这种表达与肿瘤组织和细胞内病毒基因组的物理状态(游离或整合)、肿瘤组织的分化状态(高分化或低分化)、病毒基因组插入宿主基因组基因序列后的状态(正常阅读或移码阅读)有着非常密切的关系。尽管本研究对肿瘤组织及其病毒进行检测的指标还不够丰富,如L1基因转录物的检测及测序、细胞分化指标等,致使我们对L1蛋白在肿瘤组织内的表达及其规律的解释和证明还缺少足够的说服力。但是我们希望通过该项研究引发人们对肿瘤组织表达L1蛋白的关注,促进L1蛋白相关肿瘤生物学的研究。
5 利益冲突
所有作者声明本文不涉及任何利益冲突。
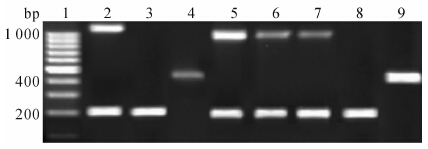
图 4 宫颈病变组织HPV16、18病毒基因组物理状态检测电泳图Fig 4 Physical state detection electrophoretogram of HPV 16,18 virus genomes in cervical lesion tissues
1: DNA marker; 2: CaSki cells were used as control of mixed HPV16 genome in which episomal genome dominated (206 bp and 1 026 bp,E2/E6>0.8); 3: SiHa cells were used as control of integrated HPV16 genome (206 bp); 4: HeLa cells were used as control of integrated HPV18 genome (452 bp); 5: Specimen was shown as positive of episomal HPV16 genome; 6,7: Specimens were shown as the mixed HPV16 genome; 8: Specimen was shown as the positive of integrated HPV16 genome; 9: Specimen was shown as the positive of integrated HPV18 genome
![]()
表 2 HPV型别鉴定及物理状态检测结果
Tab 2 HPV type determination and physical state detection
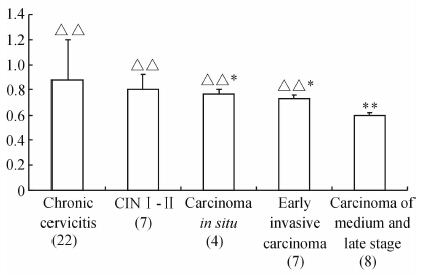
图 5 HPV阳性标本ELISA检测结果Fig 5 ELISA detection results of HPV positive specimens
The results showed that with the aggravation of lesion degree,the expression of HPV L1 declined. **P<0.01 vs cervicitis lesion tissues; △△P<0.01 vs other lesions

图 6 HPV L1蛋白在含有HPV16基因组的不同病变宫颈组织中的表达Fig 6 Western blotting analysis of HPV L1 protein expression in different cervical lesion tissues with HPV16 genome
1: Chronic cervicitis,genome was episomal; 2: CINⅠ-Ⅱ,genome was mixed; 3: Carcinoma in situ,genome was mixed; 4: Early squamous-celled carcinoma,genome was mixed; 5: Squamous-celled carcinoma of medium and late stage,genome was integrated; 6: Protein marker
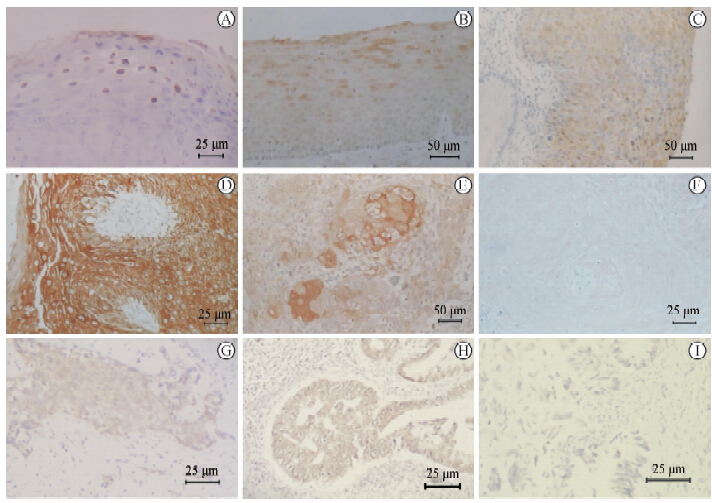
图 7 抗HPV L1免疫组织化学检测Fig 7 Analysis of anti-HPV L1 immunohistochemistry
A:Condyloma acuminate. Detection with goat anti-HPV L1 polyclonal antibody. HPV gene type was identified as HPV6. L1 positive reaction was mainly in the nucleus and rarely in the cytoplasm. The positive reaction appeared in shallow epithelium in late stage of keratinocyte differentiation. The state of tissue belonged to the combination ofⅠ-α-A and B according to Fig 2;B:Chronic cervicitis. Detection with CamVir-1 antibody. The gene type was identified as HPV16 and the genome was the in episomal state. L1 positive reaction was mainly in the cytoplasm and rarely in the nucleus. The state of tissue belonged to the combination ofⅠ-α-A and B according to Fig 2;C:Carcinoma in situ of cervix. Detection with CamVir-1 antibody. The gene type was identified as HPV16 and the genome was in the mixed state. Weakly medium L1 positive reaction could be seen in tumor tissue. Positive reactions could be seen in the cytoplasm and the nucleus. The intensity of L1 positive reaction was relatively uniform in the whole tissue. The state of tissue belonged to the combination ofⅡ-β-A according to Fig 2;D:Carcinoma in situ of cervix. Detection with Cam Vir-1 antibody. The gene type was identified as HPV16 and the genome was in the integrated state. Strong L1 positive reaction appeared in the lesion tissue. The intensity of positive reaction was relatively uniform in the whole tissue and was not related to tissue structure and differentiation. The reaction was mainly concentrated in the cytoplasm and was weaker in the nucleus. The state of tissue belonged to the combination of Ⅲ-γ-A and B according to Fig 2;E:Moderately and highly differentiated squamous carcinoma in the middle or advanced stage in the cervix uteri. Detection with Cam Vir-1 antibody. The gene type was identified as HPV16 and the genome was in mixed state. In the zone of keratin pearls where tumor cells were clearly differentiated,L1 reaction was greatly stronger than that in the surrounding tissue where tumor cells were lowly differentiated. The state of tissue belonged to the combination of Ⅱ-γ-A and B according to Fig 2;F: Moderately and high differentiated squamous carcinoma in the middle and advanced stage in cervix uteri. Detection with mice anti-HPV L1 polyvalent monoclonal antibody. The gene type was identified as HPV16 and the genome was in integrated state. The L1 positive reaction was negative or very weakly positive and the reaction intensity associated with the differentiateion degree of tumor cells. The state of tissue belonged to the combination of Ⅲ-γ-A and B according to Fig 2; G: Moderately differentiated squamous carcinoma in the middle or advanced stage in cervix uteri. Detection with mouse anti-HPV L1 polyvalent monoclonal antibody. The gene type was identified as HPV16 and the genome was in the integrated state. The L1 reaction was at moderate intensity level and the intensity was similar in the scattered tumor cells and tumor tissue. The state of tissue belonged to the combination of Ⅲ-γ-B according to Fig 2; H: Moderately differentiated adenocarcinoma in the middle or advanced stage in cervix uteri. Detection with mouse anti-HPV L1 polyvalent monoclonal antibody. The gene type was identified as HPV18 and the genome was in the integrated state. The L1 reaction was at moderate intensity level and the intensity was similar in tumor tissue. The state of tissue belonged to the combination of Ⅲ-γ-B according to Fig 2; I: Moderately and poorly differentiated adenocarcinoma in the middle or advanced stage in cervix uteri. Detection with mouse anti-HPV L1 polyvalent monoclonal antibody. The gene type was identified as HPV18 and the genome was in integrated state. No obvious L1 positive reaction was seen in tumor tissue. The state of tissue belonged to the combination of Ⅲ-γ-B according to Fig 2
| [1] | Schiller J T,Castellsagué X,Garland S M.A review of clinical trials of human papillomavirus prophylactic vaccines[J].Vaccine,2012,30(Suppl 5):F123-F138. |
| [2] | Thönes N,Müller M.Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes[J].Virology,2007,369:375-388. |
| [3] | Doorbar J,Quint W,Banks L,Bravo I G,Stoler M,Broker T R,et al.The biology and life-cycle of human papillomaviruses[J].Vaccine,2012,30(Suppl 5):F55-F70. |
| [4] | Doorbar J.The papillomavirus life cycle[J].J Clin Virol,2005,32(Suppl 1):7-15 |
| [5] | Zheng Z M,Baker C C.Papillomavirus genome structure,expression,and post-transcriptional regulation[J].Front Biosci,2006,11:2286-2302. |
| [6] | 时艳梅, 肖长义, 叶 红, 王雅琴, 吴江锋.临床宫颈组织标本HPV16 L1蛋白检测[J].基础医学与临床, 2010,30:807-810 |
| [7] | Lee H,Lee K J,Jung C K,Hong J H,Lee Y S,Choi Y J,et al.Expression of HPV L1 capsid protein in cervical specimens with HPV infection[J].Diagn Cytopathol,2008,36:864-867. |
| [8] | Yoshida T,Sano T,Kanuma T,Owada N,Sakurai S, Fukuda T,et al.Immunochemical analysis of HPV L1 capsid protein and p16 protein in liquid-based cytology samples from uterine cervical lesions[J].Cancer,2008,114:83-88. |
| [9] | Corden S A,Sant-Cassia L J,Easton A J,Morris A G.The integration of HPV-18 DNA in cervical carcinoma[J].Mol Pathol,1999,52:275-282. |
| [10] | Peitsaro P,Johansson B,Syrjänen S.Integrated human papillomavirus type 16 is frequently found in cervical cancer precursors as demonstrated by a novel quantitative real-time PCR technique[J].J Clin Microbiol,2002,40:886-891. |
| [11] | Wentzensen N,Ridder R,Klaes R,Vinokurova S, Schaefer U,Doeberitz Mv.Characterization of viral-cellular fusion transcripts in a large series of HPV16 and 18 positive anogenital lesions[J].Oncogene,2002,21:419-426. |
| [12] | Baker C C,Phelps W C,Lindgren V,Braun M J,Gonda M A,Howley P M.Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines[J].J Virol,1987,61:962-971. |
| [13] | Boshart M,Gissmann L,Ikenberg H,Kleinheinz A, Scheurlen W,zur Hausen H.A new type of papillomavirus DNA,its presence in genital cancer biopsies and in cell lines derived from cervical cancer[J].EMBO J,1984,3:1151-1157. |
| [14] | 姜波玲, 肖长义, 袁太宁, 叶 红, 王雅琴, 黎家华, 等.HPV L1 C-末端保守序列短肽体液免疫学特性[J].免疫学杂志,2009,25:239-242,246. |
| [15] | Zhang T,Xu Y,Qiao L,Wang Y,Wu X,Fan D,et al.Trivalent Human Papillomavirus (HPV) VLP vaccine covering HPV type 58 can elicit high level of humoral immunity but also induce interference among component types[J].Vaccine,2010,28:3479-3487. |
| [16] | Wu T C,Hsieh S T,Purow B W,Kurman R J.Demonstration of human papillomavirus (HPV) genomic amplification and viral-like particles from CaSki cell line in SCID mice[J].J Virol Meth,1997,65:287-298. |
| [17] | Yu T,Ferber M J,Cheung T H,Chung T K,Wong Y F,Smith D I.The role of viral integration in the development of cervical cancer[J].Cancer Genet Cytogenet,2005,158:27-34. |
| [18] | Macville M,Schräck E,Padilla-Nash H,Keck C, Ghadimi B M,Zimonjic D,et al.Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping[J].Cancer Res,1999,59:141-150. |
| [19] | Zhao X,Fay J,Lambkin H,Schwartz S.Identification of a 17-nucleotide splicing enhancer in HPV-16 L1 that counteracts the effect of multiple hnRNP A1-binding splicing silencers[J].Virology,2007,369:351-363. |
| [20] | Wang X,Meyers C,Wang H K,Chow L T,Zheng Z M.Construction of a full transcription map of human papillomavirus type 18 during productive viral infection[J].J Virol,2011,85:8080-8092. |
| [21] | 李慧弘, 肖长义, 袁太宁, 许雪梅, 黎家华, 李红军.宫颈癌HeLa细胞内HPV L1蛋白表达的观察[J].中国免疫学杂志,2013,29:260-264, 270. |
| [22] | Davidson E J,Sehr P,Faulkner R L,Parish J L,Gaston K,Moore R A,et al.Human papillomavirus type 16 E2- and L1-specific serological and T-cell responses in women with vulval intraepithelial neoplasia[J].J Gen Virol,2003,84(Pt 8):2089-2097. |
| [23] | Van Doornum G J,Korse C M,Buning-Kager J C,Bonfrer J M,Horenblas S,Taal B G,et al.Reactivity to human papillomavirus type 16 L1 virus-like particles in sera from patients with genital cancer and patients with carcinomas at five different extragenital sites[J].Br J Cancer,2003,88:1095-1100. |
| [24] | Jeong N H,Woo M K,Lee N W,Hur S J,Choi K S,Kim H J.Human papillomavirus 16 and 18 L1 Serology in Korean women with high-grade cervical intraepithelial neoplasia and cervical cancer[J].Arch Pharm Res,2009,32:1013-1018. |
 2014, Vol. 35
2014, Vol. 35


