2. 第二军医大学长海医院消化内科, 上海 200433
2. Department of Gastroenterology, Changhai Hospital, Second Military Medical University, Shanghai 200433, China
应激性生活事件被认为与消化系统最常见的慢性疾病,如功能性胃肠道病症(FGID)、炎症性肠病(IBD)、胃食管反流病(GERD)和消化性溃疡(PU)等的发病或症状加重相关;急、慢性应激可以引起肠道显著的病理生理改变[1]。迄今为止应激引起肠道功能障碍的具体机制尚不清楚,但肠黏膜屏障功能障碍是其可能的一种机制[2]。 二胺氧化酶(diamine oxidase,DAO)在人类小肠黏膜上层绒毛中有较高的含量和活性,肠黏膜细胞受损、坏死后可被释放入血或随坏死脱落的细胞进入肠腔内,导致其活性在血浆和肠腔增高而在肠黏膜内降低,故DAO浓度在外周血中的变化可间接反映肠黏膜屏障的完整性和损伤程度[3]。上皮细胞紧密连接(tight junction,TJ)位于上皮细胞膜外侧的顶部,分隔细胞顶面与基侧面,防止上皮细胞间隙中的物质溢出和大分子在细胞间隔中穿行; 细胞间的TJ破坏可能导致肠上皮对大分子物质的通透性增加[4]。在炎症、肠缺血-再灌注损伤、细菌毒素、应激等作用下,TJ会产生迅速的调节反应[6,7]。Occludin是第一个被发现的组成TJ的蛋白,是TJ的主要成分[8],在维持上皮细胞屏障功能方面具有重要作用。本实验主要通过观察微生态制剂 低聚半乳糖(galacto-oligosaccharides,GOS)早期干预对冷束缚应激(cold restrain stress,CRS)大鼠肠黏膜通透性及肠黏膜上皮TJ蛋白occludin表达的影响,初步探讨其作用机制。
1 材料和方法 1.1 材料
健康雄性Wistar大鼠40只,体质量150~160 g,由中国科学院上海实验动物中心提供[SCXK(沪)2002-0006]。GOS(西安德施普生物制品有限公司),冷束缚大鼠固定器(苏杭实验动物仪器厂),日立H800透射电镜(第二军医大学电镜室),TaKaRa RNA PCR试剂盒[宝生物工程(大连)有限公司],兔抗occludin一抗(Zymed公司),鼠兔通用型二抗(上海增健生物科技有限公司),RNA-direct SYBR○ R Green Realtime PCR Master Mix[东洋纺(上海)生物科技有限公司],DAO试剂购自Sigma公司。
1.2 实验动物分组40只雄性Wistar大鼠适应性喂养1周,自由饮水及进食,随后被随机分为4组: 正常对照组、GOS组、CRS组和CRS+GOS组,每组10只。实验开始前大鼠禁食24 h,自由饮水。 正常对照组大鼠在笼中自由活动,仅接受基础饮食(basal diet)、无菌水饲养;GOS组大鼠每天的饮水中添加GOS (4 g/kg)[2,9],共10 d,余处理同对照组;CRS组大鼠于普通喂养第10天,在乙醚轻度麻醉状态下被固定于专门的器具中,活动受限,置于4℃冰箱,3 h后解除束缚,将大鼠放回鼠笼;CRS+GOS组大鼠每天的饮水中添加GOS (4 g/kg),于喂养后第10天,采用与CRS组相同的方法制备模型。实验结束后予1%戊巴比妥钠腹腔注射麻醉,心脏取血2 mL,分离血清待测;取血后处死大鼠,取回肠组织待测。
1.3 血浆DAO浓度检测配制DAO标准品后,进行标本检测。在试管中依次加入 0.2 mol/L PBS 3.0 mL、0.004% 辣根过氧化物酶(HRP)0.1 mL、0.5%二盐酸邻联茴香胺(o-dianisidine)0.1 mL、血浆0.5 mL、0.175%戊二胺0.1 mL。 空白管用PBS液代替样本。置37℃水浴30 min,在DU-7型紫外可见光分光光度计波长436 nm处测定光密度值,以DAO标准品倍比稀释建立标准曲线,将检测结果与之对比,计算DAO含量[5]。
1.4 组织病理学改变(H-E染色法)回肠组织经生理盐水冲洗后按以下步骤进行病理检测: 4%甲醛固定24 h,梯度乙醇脱水、二甲苯透明,石蜡包埋,组织切片机切成3~4 μm切片,37℃烤片4 h,石蜡切片脱蜡至水,苏木精染色5~10 min,自来水冲洗3~4 min,75%乙醇盐酸分化,温热水5~10 min显蓝,95%乙醇冲洗1 min,伊红复染2 min,脱水透明(95%乙醇和纯乙醇脱水,二甲苯各2次,每次2 min),树脂封固,镜检观察。
1.5 透射电镜检测对切取的大鼠回肠组织,先用4%多聚甲醛固定24 h,再切成1 mm×2 mm大小,放入固定液中;0.1 mol/L的PBS液漂洗,1%四氧化锇固定液4℃下固定2 h,再以PBS液漂洗;梯度乙醇、丙酮脱水,再用11丙酮/包埋剂浸透2 h,12丙酮/包埋剂浸透过夜,纯包埋剂浸透1 h;聚合(37℃12 h,60℃ 36 h);切片经饱和醋酸铀、柠檬酸铅染色,在光镜下半薄切片定位观察,再用超薄切片机切片,常规染色后在透射电镜下观察、拍照。
1.6 小肠黏膜occludin在蛋白及基因水平的表达检测 1.6.1 免疫组织化学染色回肠组织标本经二甲苯脱蜡和梯度乙醇水化,PBS洗3次,微波抗原修复;再以PBS漂洗,3%H2O2孵育5 min,PBS漂洗,1%小牛血清孵育20 min;滴加兔抗occludin一抗 (1400稀释),4℃孵育过夜;PBS漂洗,滴加鼠兔通用型二抗50 μL,室温静置30 min;PBS漂洗,DAB显色1 min;苏木精复染、盐酸乙醇分化;晾干、滴加树脂封片、镜检。将免疫组化染色片在0.5 cm×1.0 cm样品截面上,用计算机图像处理系统进行定量灰度扫描,200倍放大系数下,每个切片随机挑选5个视野,求出给定面积的灰度值。灰度值范围为0~255,染色越深,灰度越低。运用ImaginPro Plus软件计算并比较各自表达量所对应的灰度值,用于反映occludin蛋白的相对表达水平(灰度值低代表蛋白表达水平高)。
1.6.2 实时荧光定量PCR检测取回肠组织进行RNA抽提,取5 μg行电泳以检测RNA纯度和浓度。使用TaKaRa RNA PCR试剂盒将RNA反转录为cDNA,保存于4℃冰箱。使用Primer Premier 5.0软件设计引物序列,由上海生化生物工程公司合成,分别是: β-actin,引物序列为 5′-CCT GTA CGC CAA CAC AGT GC-3′、 5′-ATA CTC CTG CTT GCT GAT CC-3′,退火温度56℃,产物长度211 bp;occludin: 引物序列为 5′-AAA CCC GAA GAA AGA TGG AC C-3′、 5′-TCA CTT TGC CGT TGG AGG AG-3′,退火温度58℃,产物长度198 bp。建立25 μL的实时PCR反应体系,用ABI 7500 PCR仪进行扩增。荧光定量PCR的结果以Ct值显示,采用相对定量2-ΔΔCt法比较各基因表达的差异。
1.7 统计学处理数据采用SPSS 12.0软件进行统计分析。所有实验数据均以x±s表示,各组之间的比较采用方差分析,两组间比较采用配对t检验。检验水准(α)为0.05。
2 结 果2.1 血浆DAO浓度
CRS组、CRS+GOS组大鼠血浆DAO浓度分别为 (24.75±2.73) U/mL、(17.36±1.50) U/mL,均明显高于正常对照组 (8.42±2.58) U/mL,P<0.01),CRS组血浆DAO浓度又明显高于CRS+GOS组(P<0.01),但血浆DAO浓度在正常对照组和GOS组(9.10±2.31) U/mL之间差异无统计学意义(P>0.05)。
2.2 小肠组织病理学改变的光镜观察小肠黏膜损伤以Chiu分级法[10]为评分标准。各组大鼠小肠壁层次清楚,其中正常对照组和GOS组大鼠肠道黏膜上皮结构完整,无明显充血和异常淋巴细胞浸润,评分0级。CRS组大鼠肠黏膜变薄、萎缩,浅层有部分坏死,上皮细胞脱落,部分绒毛顶端固有层有明显充血水肿,肠黏膜上皮细胞与固有层间距离增宽,评分3级;CRS+GOS组大鼠肠黏膜变薄,无明显细胞脱落、坏死,充血水肿不明显,评分1级。结果如图 1所示。
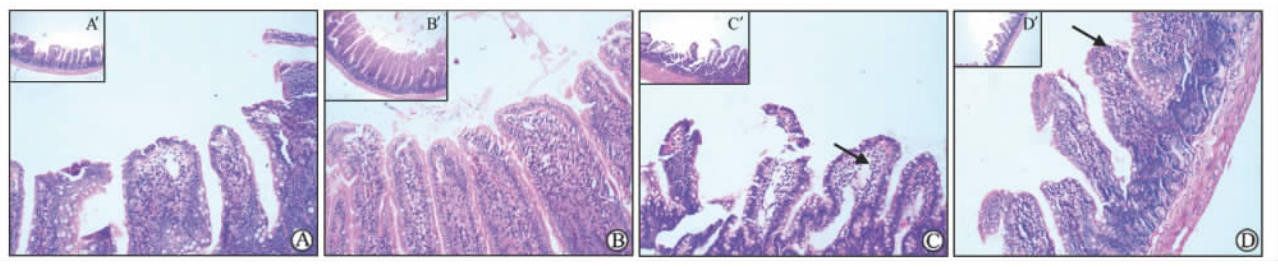 | 图 1 各组大鼠小肠组织H-E染色结果Fig 1 H-E staining of the small intestinal tissue of rats in each groupA: Normal control group; B: GOS (galacto-oligosaccharides,4 g·kg-1·d-1,×10 d) group; C: CRS (cold restrain stress) group, arrow showed the rats’ intestinal mucosa became thinned with atrophy,partial necrosis of the superficial mucosal,epithelial cells shedding and obvious edema at the lamina propria of villi top; the gap between the intestinal epithelial cells and the lamina propria became wider; D: CRS+GOS group,arrow showed the rats’ intestinal mucosa became thinner with no obvious cell shedding,necrosis or edema. A′,B′,C′,D′: Corresponding to A,B,C,and D,respectively. Original magnification: ×100 (A-D); ×40 (A′-D′) |
正常对照组和GOS组肠黏膜上皮细胞表面微绒毛排列整齐,柱状上皮细胞结构完整;细胞器结构未见异常;细胞间紧密连接、中间连接、桥粒及缝隙连接清楚,无增宽。CRS组大鼠肠段主要表现为上皮细胞微绒毛稀疏、排列不整、呈倒伏状、部分缺如;上皮细胞连接增宽、部分紧密连接增宽或开放;线粒体肿胀,内质网扩张。CRS+GOS组大鼠肠段部分上皮细胞微绒毛结构完整,细胞间连接稍增宽,有轻微溶解现象,但较之CRS组大鼠肠段超微结构的改变要微小。结果如图 2所示。
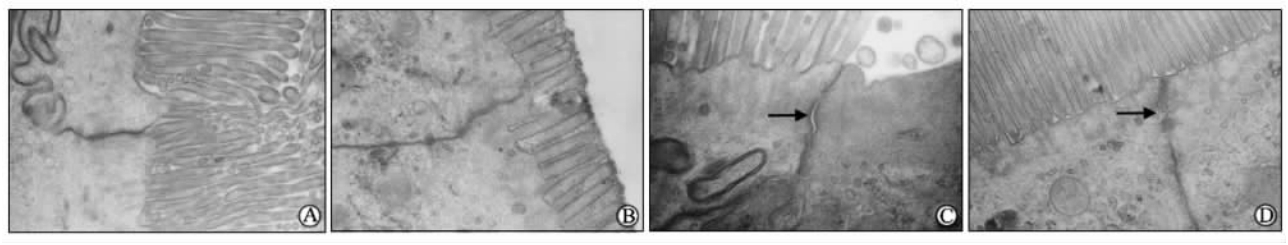 | 图 2 各组大鼠小肠绒毛顶端超微结构改变Fig 2 Ultrastructure changes of the small intestinal villus tip of rats in each groupA: Normal control group; B: GOS (galacto-oligosaccharides,4 g·kg-1·d-1,×10 d) group; C: CRS (cold restrain stress) group, arrow showed that the junctions between epithelial cells were widened and some tight junctions were widened; D: CRS+GOS group,arrow showed the cellular junctions became slightly widened,with slight dissolution phenomena. Original magnification: ×20 000 |
免疫组化染色结果显示, occludin蛋白细胞表面、细胞间、胞质内均有表达,阳性染色呈棕黄色, 正常对照组(图 3A)和GOS组(图 3B)较CRS组(图 3C)和CRS+GOS组(图 3D)染色深。相对定量分析结果显示,CRS组和CRS+GOS组平均灰度值均显著高于正常 对照组(P<0.01),CRS组又高于CRS+GOS组(P<0.01),但正常对照组和GOS组之间差异无统计学意义(P>0.05,图 3E)。说明CRS组和CRS+GOS组大鼠小肠上皮细胞间occludin蛋白表达较正常对照组均明显降低,但 CRS+GOS组的表达水平较CRS组有所增加。与此相对应的是,在occludin mRNA表达水平方面,CRS组和CRS+GOS组也较正常对照组降低(P<0.01),且CRS+GOS组表达水平高于CRS组P<0.01);正常对照组与GOS组之间差异也无统计学意义(P>0.05,图 3F)。
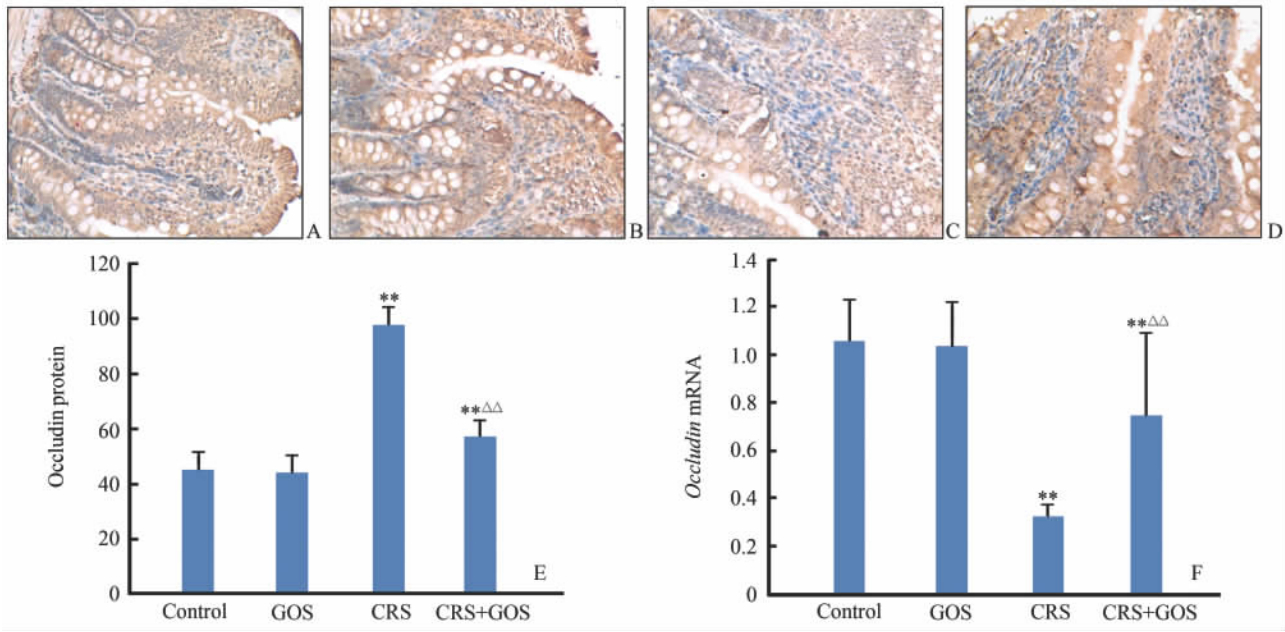 | 图 3 各组大鼠小肠黏膜occludin蛋白的免疫组化检测及 occludin mRNA的相对含量Fig 3 Expression of occludin mRNA and protein in the small intestinal mucosa of rats in each groupA-D: Immunohistochemical staining,original magnification: ×200. A: Normal control group; B: GOS (galacto-oligosaccharides,4 g·kg-1·d-1,×10 d) group; C: CRS (cold restrain stress) group; D: CRS+GOS group; E: Average gray scale value of small intestinal occludin protein; F: Expression of occludin mRNA. **P<0.01 vs control group; △△P<0.01 vs CRS group. n=10,x±s |
动物研究显示,大鼠急性束缚模型(acute restrain stress)建立成功后,在肠黏膜组织形态学方面尚未有明显改变时,即出现旁细胞通路(paracellular pathway)通透性的增高,细胞TJ开放,说明细胞间TJ的破坏是早期肠屏障功能受损,通透性增高的主要原因[11]。
Occludin蛋白作为TJ的主要成分,具有4次跨膜结构,与claudin1、claudin2及其他一些外周膜蛋白等连接共同构成上皮的紧密连接结构[12,13],在维持上皮细胞屏障功能方面具有重要作用。肠黏膜occludin蛋白及其mRNA水平的变化可以在一定程度上反映肠黏膜紧密连接及肠屏障的破坏情况。在许多炎症性肠疾病中,均发现有肠黏膜occludin水平的下降[14,15]。许多肠致病菌及其毒素也会破坏肠上皮的TJ结构、减少occludin含量,并可能与激活宿主的细胞信号转导通路有关[16,17,18]。在一项大型临床研究中发现胃肠炎水样泻后,肠易激综合征(IBS)组的肠道通透性比对照组明显增高[19]。
改善肠道的屏障功能可能是预防或改善应激诱导的肠道功能障碍的新对策,目前在肠屏障功能方面,很重要的一个研究领域就是对微生态制剂的研究。微生态制剂主要包括益生菌(probiotics)、益生元(prebiotics)及两者共同构成的合生素(synbiotics)等一系列微生态物质,它们在保护肠屏障功能方面的作用既有共性,又有区别。长期以来,国内外学者对益生菌的研究较多,而关于益生元的研究才刚起步,很多研究还处于空白。常见的益生元有各种低聚糖类,如: 低聚果糖、低聚木糖、GOS、低聚麦芽糖等。研究表明,益元生可能通过抑制病原微生物、免疫调节、促进有机酸生成、营养上皮细胞的机制来保护肠道屏障[20,21]。
体外采用单层肠上皮细胞进行研究显示,在受E.coli作用的小肠上皮细胞反应体系中加入乳酸杆菌等益生菌可以避免TJ破坏,增加occludin及细胞骨架蛋白的表达[22]。我们的研究结果显示,与对照组相比,各CRS组大鼠小肠上皮细胞间occludin蛋白表达和mRNA水平均明显降低,CRS+GOS组的occludin蛋白表达和mRNA水平均高于CRS组, 提示添加GOS可以增加occludin mRNA和蛋白表达水平,从而改善紧密连接和维护肠屏障功能。
目前的研究发现应激与一些消化道常见疾病密切相关,但具体机制不明确,预防和治疗仍缺乏有效的手段。因此,与此相关的研究是目前研究的热点。微生态制剂用于应激反应性疾病的防治还有许多亟待解决的问题,其应用前景值得观察和期待。
4 利益冲突所有作者声明本文不涉及任何利益冲突。
| [1] | Konturek P C,Brzozowski T,Konturek S J.Stress and the gut:pathophysiology,clinical consequences,diagnostic approach and treatment options[J].J Physiol Pharmacol,2011,62:591-599. |
| [2] | Cameron H L,Perdue M H.Stress impairs murine intestinal barrier function:improvement by glucagon-Like peptide-2[J].J Pharmacol Exp Ther,2005,314:214-220. |
| [3] | Tsunooka N,Maeyama K,Hamada Y,Imagawa H,Takano S,Watanabe Y,et al.Bacterial translocation secondary to small intestinal mucosal ischemia during cardiopulmonary bypass.Measurement by diamine oxidase and peptidoglycan[J].Eur J Cardiothorac Surg,2004,25:275-280. |
| [4] | Soderholm J D,Perdue M H.Stress and gastrointestinal tract.Ⅱ.Stress and intestinal barrier function [J].Am J Physiol Gastrointest Liver Physiol,2001,280:G7-G13. |
| [5] | 黎君友,于 燕,郝 军,晋 桦,许惠君.分光光度法测定血和小肠组织二胺氧化酶活性[J].氨基酸和生物资源,1996,18:28-30. |
| [6] | Fasano A,Nataro J P.Intestinal epithelial tight junctions as targets for enteric bacteria-derived toxins[J].Adv Drug Deliv Rev,2004,56:795-807. |
| [7] | Inoue K,Oyamada M,Mitsufuji S,Okanoue T,Takamatsu T.Different changes in the expression of multiple kinds of tight-junction proteins during ischemia-reperfusion injury of the rat ileum[J].Acta Histochem Cytochem,2006,39:35-45. |
| [8] | González-Mariscal L,Betanzos A,Nava P,Jaramillo B E.Tight junction protein[J].Prog Biophys Mol Biol,2003,81:1-44. |
| [9] | Rahman S H,Ammori B J,Holmfield J,Larvin M,McMahon M J.Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis[J].J Gastrointest Surg,2003,7:26-36. |
| [10] | Chiu C J,McArdle A H,Brown R,Scott H J,Gurd F N.Intestinal mucosal lesion in low-flow states. Ⅰ. A morphological,hemodynamic,and metabolic reappraisal[J].Arch Surg,1970,101:478-483. |
| [11] | Mazzon E,Sturniolo G C,Puzzolo D,Frisina N,Fries W.Effect of stress on the paracellular barrier in the rat ileum[J].Gut,2002,51:507-513. |
| [12] | Anderson J M.Molecular structure of tight junctions and their role in epithelial transport[J].News Physiol Sci,2001,16:126-130. |
| [13] | Tsukita S,Furuse M,Itoh M.Multifunctional strands in tight junctions[J].Nat Rev Mol Cell Biol,2001,2:285-293. |
| [14] | Ara N,Iijima K,Asanuma K,Yoshitake J,Ohara S,Shimosegawa T,et al.Disruption of gastric barrier function by luminal nitrosative stress:a potential chemical insult to the human gastro-oesophageal junction[J].Gut,2008,57:306-313. |
| [15] | Ivanov A I,Nusrat A,Parkos C A.The epithelium in inflammatory bowel disease:potential role of endocytosis of junctional proteins in barrier disruption[J].Novartis Found Symp,2004,263:115-124. |
| [16] | Howe K L,Reardon C,Wang A,Nazli A,McKay D M.Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function andblocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability[J].Am J Pathol,2005,167:1587-1597. |
| [17] | Chen M L,Ge Z,Fox J G,Schauer D B.Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni[J].Infect Immun,2006,74:6581-6589. |
| [18] | Shifflett D E,Clayburgh D R,Koutsouris A,Turner J R,Hecht G A.Enteropathogenic E.coli disrupts tight junction barrier function and structure in vivo[J].Lab Invest,2005,85:1308-1324. |
| [19] | Marshall J K,Thabane M,Garg A X,Clark W,Meddings J,Collins S M,et al.Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton,Ontario[J].Aliment Pharmacol Ther,2004,20(11-12):1317-1322. |
| [20] | Ouwehand A C,Derrien M,de Vos W,Tiihonen K,Rautonen N.Prebiotics and other microbial substrates for gut functionality[J].Curr Opin Biotechnol,2005,16:212-217. |
| [21] | 杨建军,耿 翔,高志光,秦环龙.益生菌及肠内外营养对重症急性胰腺炎大鼠肠道黏附分子及免疫屏障的影响[J].世界华人消化杂志,2006,14:953-957. |
| [22] | Hirano J,Yoshida T,Sugiyama T,Koide N,Mori I,Yokochi T.The effect of lactobacillus rhamnosus on enterohemorr hagic Escherichia coli infection of human intestinal cells in vitro[J].Microbiol Immunol,2003,47:405-409. |
 2014, Vol. 35
2014, Vol. 35


