扩展功能
文章信息
- 周锡强, 陈代钊, 刘牧, 胡建芳
- ZHOU XiQiang, CHEN DaiZhao, LIU Mu, HU JianFang
- 中国沉积学发展战略:沉积地球化学研究现状与展望
- The Future of Sedimentology in China: A Review and Perspective of Sedimentary Geochemistry
- 沉积学报, 2017, 35(6): 1293-1316
- ACTA SEDIMENTOLOGICA SINCA, 2017, 35(6): 1293-1316
- 10.14027/j.cnki.cjxb.2017.06.020
-
文章历史
- 收稿日期:2016-10-02
- 收修改稿日期: 2016-10-20
2. 中国科学院地球科学研究院, 北京 100029;
3. 中国科学院大学, 北京 100049;
4. 中国科学院广州地球化学研究所, 有机地球化学国家重点实验室, 广州 510640
2. Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China;
4. State Key Laboratory of Organic Geochemistry, Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China
沉积地球化学是沉积学与地球化学相互渗透、并结合了环境地球化学、生物地球化学、海洋地球化学等研究内容的交叉学科,通过元素地球化学、同位素地球化学、有机地球化学等研究手段、方法与理论,分析地球表层沉积岩(物)的化学作用、化学组成及化学演化,揭示沉积物质的来源、分布、转化和循环过程,并解析相关的资源环境效应及表层系统圈层相互作用。目前,沉积地球化学已成为沉积学的重要研究内容和关键技术手段,并在重大地质历史时期环境与生物的演化过程,以及沉积矿产资源和化石能源的形成规律等研究方面,发挥着不可替代的作用。随着地球化学分析技术的快速发展,以及国家经济发展和科研经费投入的增加,我国沉积地球化学研究正在经历快速成长期。此报告通过文献调研和期刊数据库资料的挖掘,揭示国内外沉积地球化学研究现状,并结合国情分析我国的机遇与挑战,为学科发展战略提供有益的参考和建议。
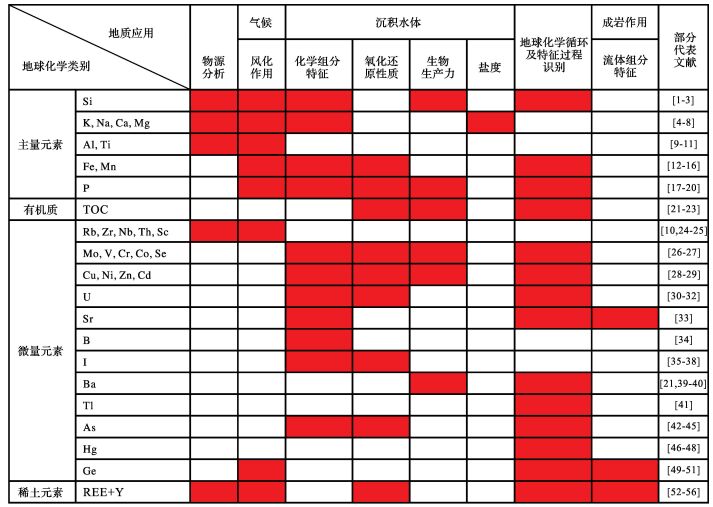
1 沉积地球化学发展现状 1.1 沉积地球化学分析测试技术沉积地球化学发展基础依赖于对沉积岩(物)及相关流体的元素、同位素及有机化合物的精确分析。近30年来,地球化学分析测试技术在经济、高效、高精准度、高时空分辨率等方面取得了巨大进步,极大地促进了沉积地球化学方法、理论及应用的发展。目前,各类质谱仪、光谱仪、色谱仪、电子探针和扫描电镜等仪器,可对多种元素、同位素及有机化合物进行高精度测试分析,已经广泛应用于沉积地球化学研究(表 1)。其中,质谱仪结合激光剥蚀或二次离子技术、扫描电镜结合能谱仪、原位微区X射线荧光光谱仪、场发射电子探针、及激光拉曼仪等,可对沉积岩(物)固体样品进行原位微区成分分析与扫描成像,具有广阔的应用前景。同时,样品预处理平台可为沉积地球化学分析提供必要的支持。
| 仪器类别 | 主要用途 | |
| 样品预处理平台 | 无污染碎样平台、单矿物分选平台、微区取样技术平台、化学预处理平台(如超净实验室)等 | 样品预处理 |
| 质谱仪 | 电感耦合等离子体质谱(ICP-MS) | 元素和同位素分析(及分布图像),如微量和稀土元素,稳定及非传统同位素,U-Th、U-Pb、Pb-Pb、Rb-Sr、Sm-Nd、Re-Os、PGE等 |
| 多接收—电感耦合等离子体质谱(MC-ICP-MS)+激光剥蚀系统(LA) | ||
| (纳米)二次离子质谱仪(Nano-SIMS) | ||
| 热电离质谱仪(TIMS)+同位素稀释法(ID) | ||
| 气体同位素质谱仪 | C、H、O、N、S等稳定同位素分析 | |
| 稀有气体质谱仪 | He、Ar等稀有气体同位素分析 | |
| 光谱仪 | 电感耦合等离子体发射光谱仪(ICP-OES) | 常量及微量元素的定量分析 |
| X射线荧光光谱仪(XRF) | ||
| 原位微区X射线荧光光谱仪(Micro-XRF) | ||
| 原子吸收光谱仪(AAS) | ||
| X射线衍射光谱仪(XRD) | 矿物相定性及半定量分析 | |
| 激光共焦显微拉曼光谱仪(LCM-Raman) | 微区成分及矿物分析 | |
| 色谱仪 | 气相色谱仪(GC) | 有机化合物定性—定量分析 |
| 气相色谱/质谱联用仪(GC-MS) | ||
| 离子色谱仪(IC) | 离子定量分析 | |
| 探针—电镜(能谱)类 | 高分辨场发射电子探针(FE-EPMA) | 微区成分和矿物分析(及成像)等 |
| 场发射扫描电子显微镜(FE-SEM)+能谱仪(EDS) | ||
| 聚焦离子束—扫描电子显微镜(FIB-SEM) | ||
| 矿物分析—场发射扫描电镜(AMICSCAN) | ||
| 其他 | 碳氢氮硫分析仪 | C、H、N、S定量分析 |
目前,中国已拥有多套国际一流的地球化学分析测试仪器,主要配置于中国科学院下属的研究所(如地质与地球物理、地球化学、广州地球化学、地球环境、南京地质古生物等研究所),中国地质科学院,教育部直属高校(如北京大学、南京大学、中国地质大学、中国石油大学等),以及企业的研究院所(如中国石油勘探开发研究院、核工业北京地质研究院等)相关实验室均可为沉积地球化学研究提供必要的公共分析测试平台支持。然而,中国沉积地球化学的测试分析仍面临实验室机时紧张、测试费用较高、测试方法和适用对象有限等不利条件,制约着该学科在技术方法和理论方面的发展与创新,以及相关研究应用的深化和推广。
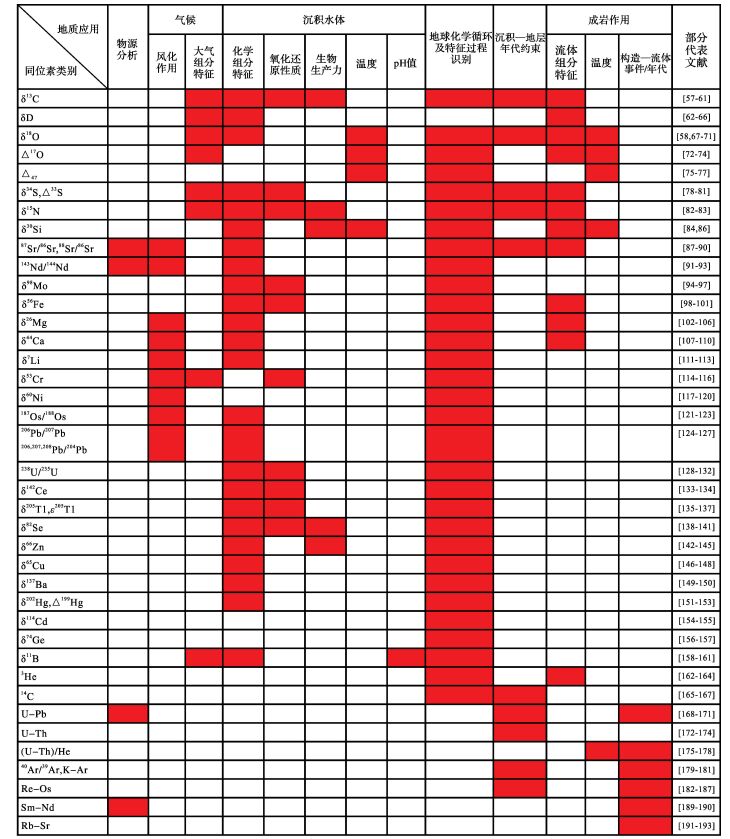
1.2 地球化学在沉积学研究中的应用沉积岩(物)经历了风化、搬运、沉积和成岩作用等一系列过程,是陆源物质、生物物质、水柱自生矿物及成岩矿物的组合。沉积岩(物)的化学组分不同程度的响应了物源输入,气候背景(大气组分特征及风化作用),沉积水体(氧化还原性质、特征化学组分及含量、生物生产力、温度、pH值、盐度等),生物类别(如高等植物、藻类、细菌等),地球化学循环与特征过程(如物源—气候—环境—生物等变化、及火山—构造—热液等事件),以及成岩作用(构造—流体事件及年代学、成岩流体的化学特征与温度)。因此,沉积岩(物)是地球表层系统圈层相互作用的记录者,通过元素、同位素的地球化学行为及有机化合物特征,可以示踪相关的地质过程(表 2,3,4)。近年来,地球化学理论、方法和技术取得了长足进步,极大的提升了对沉积岩(物)的分析能力,丰富了沉积学与沉积地球化学的研究内容,拓展了对地球表层系统演化及资源环境效应的研究广度和深度。
 |
主量元素(Si、K、Na、Ca、Mg、Al、Ti、Fe、Mn、P)含量高、易测试,常用于物源分析、风化作用、水体化学组分特征、元素循环及特征地质过程识别等方面的研究(表 2)。其中,Fe、Mn元素广泛用于示踪水体氧化还原性质,K、Na、Ca、Mg等元素可用于重建水体盐度特征。此外,TOC(总有机碳含量)、P、及Si(硅藻类生源组分)也常用于生物生产力研究。
微量元素含量较低,但类型丰富,资源及环境意义显著,是沉积地球化学的重要研究对象(表 2)。其中,Mo、V、Cr、Co、Se、Cu、Ni、Zn、Cd、U等元素的富集—亏损情况可较好示踪沉积环境,尤其是水体氧化还原性质或生物生产力的变化,并有助于识别特征地质过程。另一方面,Ba元素则常作为生产力相关的指标。B、I、Tl、Hg、Ge、As等元素的沉积地球化学循环及其环境效应也受到广泛关注。此外,Rb、Zr、Nb、Th、Sc等元素一定程度上可用于解析物源和风化作用。总之,微量元素的地球化学循环过程及时空演化特征有助于揭示沉积环境性质和特征地质过程,具有广阔的应用前景。
稀土元素(REE+Y)的离子半径相近、化学性质相似,但在一定地质条件下,可产生显著的分异。在沉积地球化学研究中,REE+Y的含量特征,以及经标准化的分异特征,可用于揭示物源类型、风化作用、水体氧化还原性质(如Ce异常)、特征地质过程(如Eu异常)、及成岩流体示踪等,是常用的研究方法(表 2)。
近年来,同位素地球化学快速发展,极大的丰富和拓展了沉积地球化学的研究手段与内容(表 3)。其中,δ13C、δD、δ18O、δ15N、δ34S、87Sr/86Sr等同位素在古气候、古海洋、古环境、及成岩作用等研究方面应用广泛,也是建立化学地层的常用手段。另一方面,各种传统或非传统同位素研究方兴未艾,具有广阔的开发及应用前景。其中,δ98Mo、δ56Fe、δ53Cr、238U/235U、δ142Ce、ε205Tl、δ82Se等同位素可用于示踪海洋氧化还原状态,87Sr/86Sr、143Nd/144Nd、δ26Mg、δ44Ca、δ7Li、δ53Cr、δ60Ni、187Os/188Os、206Pb/207Pb等同位素可用于评估陆地风化作用(如风化程度、类型和陆源输入通量等),δ15N、δ82Se、δ66Zn等同位素可用于指示海洋生物生产力变化。此外,上述以及其他同位素(如δ30Si、88Sr/86Sr、δ137Ba、δ202Hg,Δ199Hg、δ114Cd、δ74Ge、3He)的元素循环过程,以及对特征地质过程的识别,也是目前沉积地球化学的重要研究内容与方法。值得注意的是,近年来,δ18O可作为古高度计或温度计,Δ47(团簇同位素)可约束流体温度,Δ33S可约束早期大气组分特征,δ11B可约束水体pH值和大气pCO2,具有特殊的应用效力和广阔的发展前景。此外,14C、U-Pb、U-Th、Re-Os、40Ar/39Ar、K-Ar等同位素可对不同材料进行不同时间尺度和精度的年代学分析,是约束沉积地层年代的关键方法。同时,(U-Th)/He、Sm-Nd、Rb-Sr等同位素可用于约束成岩作用阶段的构造—流体事件及年代,得到一定的应用。此外,碎屑锆石U-Pb、碎屑磷灰石Sm-Nd同位素也是物源分析的重要手段。
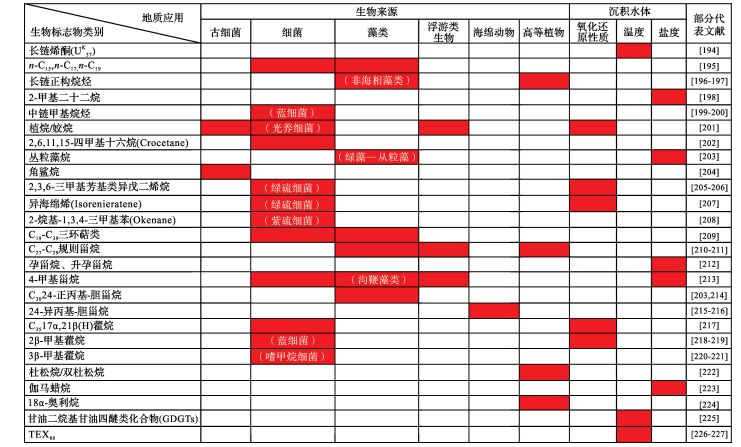
生物标志化合物是有机地球化学研究的核心内容,也是示踪沉积岩(物)的生物来源及沉积环境的重要手段(表 4)。在生物来源研究方面,正构烷烃广泛分布于各种生物体,是最为常见的生物标志物。例如,n-C15, n-C17, n-C19正构烷烃的选择性富集可反映菌藻类的优势贡献,长链正构烷烃可指示陆源高等植物的输入。同时,蓝细菌的中链甲基烷烃和2β-甲基藿烷,绿藻—从粒藻的丛粒藻烷,绿硫细菌的2, 3, 6-三甲基芳基类异戊二烯烷和异海绵烯(Isorenieratene),紫硫细菌的2-烷基-1, 3, 4-三甲基苯(Okenane),沟鞭藻类的4-甲基甾烷,嗜甲烷细菌的3β-甲基藿烷,海绵动物的24-异丙基-胆甾烷等,均是各自的特征生物标志物,在研究实践中得到了广泛应用。此外,细菌的2, 6, 11, 15-四甲基十六烷(Crocetane)、C35 17α, 21β(H)藿烷,藻类的C19~C39三环萜类、C27~C29规则甾烷、及C30 24-正丙基—胆甾烷,古细菌的角鲨烷,高等植物的长链正构烷烃、杜松烷/双杜松烷、及18α-奥利烷等,也常用于指示特定的生物类型。在沉积环境研究方面,长链烯酮类化合物(U37K)、甘油二烷基甘油四醚类化合物(GDGTs)、及TEX86等可对沉积水体温度进行约束;2-甲基二十二烷、丛粒藻烷、孕甾烷、升孕甾烷、4-甲基甾烷、伽马蜡烷等化合物则可对沉积水体盐度进行约束;植烷/鮱姣烷及厌氧细菌(如绿硫细菌、紫硫细菌、嗜甲烷细菌)生物标志物可指示水体氧化还原状态;这些有机化合物在沉积学研究中得到了广泛的关注与应用。
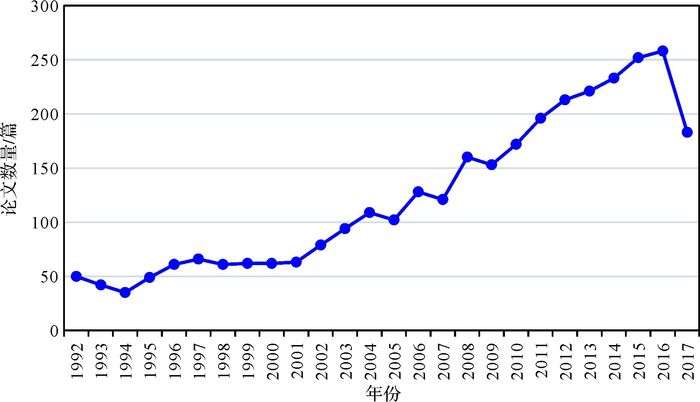
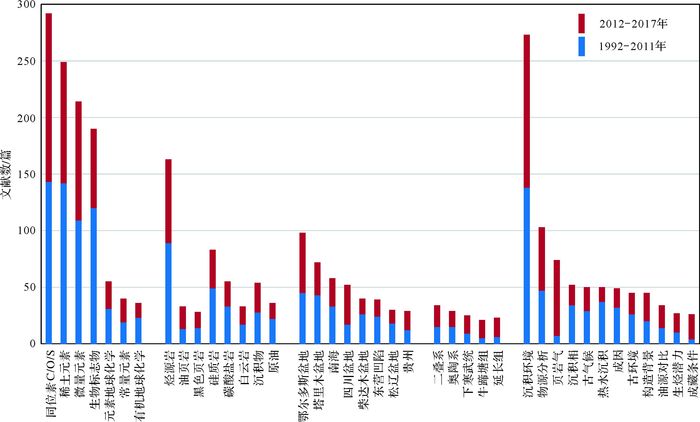
1.3 沉积地球化学的国内研究现状为了解沉积地球化学在国内的研究现状,我们基于中国知网(CNKI)的中国期刊全文数据库,对核心期刊里相关论文的发表情况进行了统计分析(截止时间为2017年9月25日)。在CNKI期刊数据库里,限定在“地质学、海洋学、资源科学、石油勘探与开采工程、环境科学”研究学科,以“沉积”(并不含“火山岩”)为主题,以“地球化学”为关键词(并分别以“稀土元素”、“微量元素”、“生物标志物”、“元素地球化学”、“有机地球化学”、“同位素”、“常量元素”等关键词进行搜索优化),检索获得SCI来源、EI来源和核心期刊论文3 230篇。通过CNKI数据库的“计量可视化分析”功能对全部检索结果进行了提取,并采用人工分析对检索结果进行了优化(如关键词的分组与合并等),获得论文“发表年度趋势”(图 1)和“关键词分布”(图 2),以追踪近25年来(1992年至2017年9月25日)沉积地球化学学科的发展历程。
|
| 图 1 国内核心期刊(基于CNKI中国学术期刊全文数据库)1992—2017年期间(截至9月25日)发表沉积地球化学相关论文的年度数量趋势 Figure 1 Annual variation of the numbers of papers on sedimentary geochemistry published in Chinese core journals from China Academic Journal Network Publishing Database of CNKI during 1992-2017 year period (by September 25) |
|
| 图 2 国内核心期刊(基于CNKI中国学术期刊全文数据库)1992—2017年期间(截至9月25日)沉积地球化学相关论文的关键词分布情况 Figure 2 The distribution of keyword frequency in articles on sedimentary geochemistry published in Chinese core journals from China Academic Journal Network Publishing Database of CNKI during 1992-2017 year period (by september 25) |
在论文发表数量方面,该研究领域在90年代总体处于平稳发展阶段,但自2000年以来呈现明显的快速增长特征(图 1),表明随着国家经济发展和科技研发经费投入的增加,沉积地球化学作为研究方法或对象,在地质学、海洋科学、环境科学及油气矿产资源等领域发挥日益重要的作用。在研究方法方面(图 2),沉积地球化学多采用稳定同位素、微量及稀土元素、生物标志物等常规或成熟手段。在研究对象方面(图 2),主要关注烃源岩(及油页岩、黑色页岩),硅质岩和碳酸盐岩(如白云岩)等,同时兼顾沉积物和原油等对象。在研究区域方面(图 2),主要集中于鄂尔多斯盆地、塔里木盆地、四川盆地、柴达木盆地、东营凹陷、松辽盆地等含油气盆地,同时南海和贵州也是重要研究区域。在研究层位方面(图 2),二叠系、奥陶系、下寒武统(及牛蹄塘组)等是主力层段,延长组近5年也吸引了较多关注。在研究内容方面(图 2),沉积环境、物源分析、古气候及古环境等是传统的重要研究内容,同时热水沉积及构造背景也具有较高的关注度;另一方面,页岩气、油源对比、生烃潜力和成藏条件等已在近5年里快速攀升为重要研究主题。
因此,通过对1992—2011年和2012—2017年期间中国沉积地球化学在研究方法、对象、区域、时代和内容的追踪可见,相关研究工作一方面紧密围绕物源分析、古环境和古气候重建等科学问题而开展,另一方面对含油气盆地及油页岩层段、及油气生储等方面保持了高度的关注,积极响应国家油气资源勘探开发的需求。
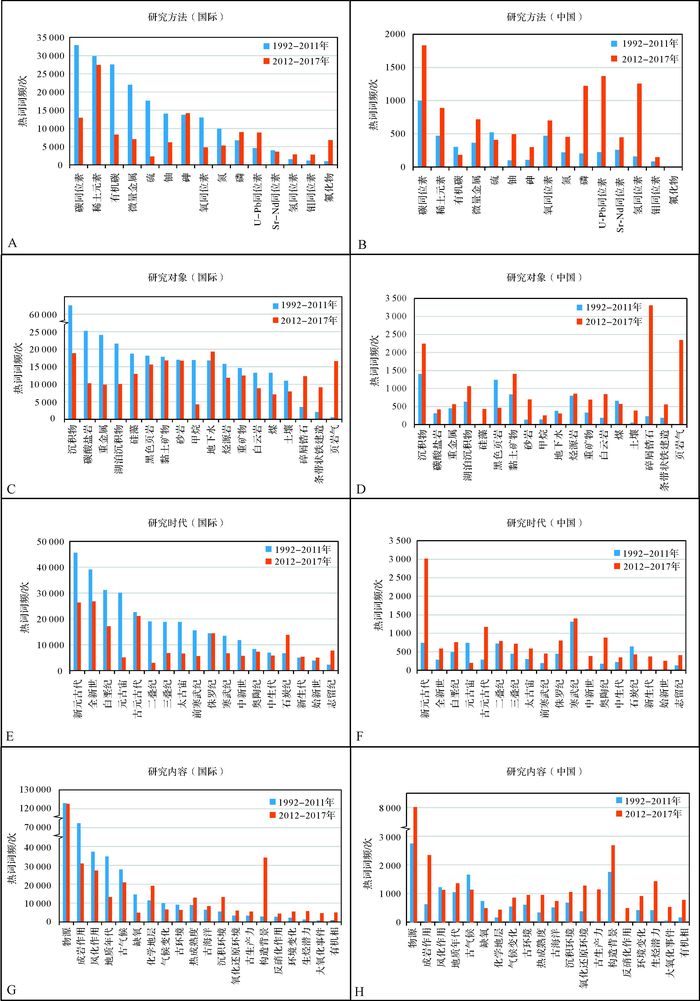
1.4 沉积地球化学的国际研究现状为综合了解中国以及国际沉积地球化学的研究现状,我们对ISI Web of Science(ISI WOS)核心合集数据库里的相关论文发表情况进行了统计分析(截止时间均为2017年9月25日)。检索时,以“sedimentary geochemistry”为“Topic”选项的检索词,同时以“lavas”、“granites”、“mantle”、“granitoid”等为“Title”选项的非检索词进行筛除,得到1997—2017年(截至9月25日)期间发表的沉积地球化学相关论文。为进一步优化检索结果,对不相关论文进行了人工筛选,最终获得4 423篇期刊论文(其中2012—2017年共1 743篇)。在此基础上,获得了主要国家在本研究领域的期刊论文“年度数量趋势”(图 3)及综合表现(表 5)。同时,论文标题和摘要里的高频学术词语(热词)一定程度上可追踪研究热度和趋势。我们将检索论文分别以国际(含中国)和中国,以及1992—2011和2012—2017(截至9月25日)时间段进行分组,通过Bibexcel软件对其高频热词进行了统计,然后以国际上1992—2011年高频热词(降序)为基准序列,对比分析国内外沉积地球化学的研究进展与动态(图 4)。

|
| 图 3 主要国家1992—2017年期间(截至9月25日)在SCIE(Web of Science核心合集)期刊里发表沉积地球化学相关论文的年度数量趋势 Figure 3 Annual variation of the numbers of papers on sedimentary geochemistry of leading countries published in journals of SCIE (Web of Science Core Collection) during 1992-2017 year period (by september 25) |
| 序 号 |
国家 | 发文量 /篇 |
总被引 次数/次 |
篇均被引 频次 /(次/篇) |
被引频次 ≥10的论文 /篇 |
被引频次 ≥30的论文 /篇 |
| 1 | 中国 | 438 | 2 514 | 5.74 | 73 | 10 |
| 2 | 美国 | 348 | 3 314 | 9.52 | 89 | 25 |
| 3 | 德国 | 169 | 1 276 | 7.55 | 38 | 6 |
| 4 | 法国 | 145 | 1 488 | 10.26 | 32 | 11 |
| 5 | 加拿大 | 136 | 1 787 | 13.14 | 41 | 12 |
| 6 | 英国 | 128 | 1 229 | 9.60 | 42 | 11 |
| 7 | 澳大利亚 | 125 | 1 134 | 9.07 | 39 | 7 |
| 8 | 印度 | 110 | 574 | 5.22 | 20 | 2 |
| 9 | 意大利 | 108 | 754 | 6.98 | 32 | 3 |
| 10 | 巴西 | 84 | 351 | 4.18 | 8 | 1 |
|
| 图 4 国际及中国1992—2017年(截至9月25日)期间沉积地球化学的研究现状及对比,基于国际SCIE期刊(Web of Science核心合集)相关论文的热词词频分布 (A)国际和(B)中国的研究方法;(C)国际和(D)中国的研究时代;(E)国际和(F)中国的研究对象;(G)国际和(H)中国的研究内容。注:国际数据包含中国。横坐标按照国际上1992—2011年期间热词频次降序排列。图C、G、H的纵坐标有部分省略 Figure 4 The state of sedimentary geochemistry studies of the world and China during 1992-2017 year period (by september 25), based on the distribution of hot word frequency in articles on sedimentary geochemistry published in journals of SCIE (Web of Science Core Collection) Sedimentary geochemical methods in related articles of the world (A) and China (B). Research materials in related articles of the world (C) and China (D). Geological periods in related articles of the world (E) and China (F). Research topics in related articles of the world (G) and China (H). Data of the World include the portion of China. The horizontal coordinates are arranged in descending order for hot word frequency of the World during 1992-2001 year period. Note the scale change of vertical coordinate in Figs C, G and H |
在文章数量方面(图 3),发表沉积地球化学论文最多的前6个国家依次是:美国、中国、德国、法国、英国和加拿大。美国论文年度发表数量常年占据领先地位,德国、法国、英国和加拿大则相对保持稳定。值得注意的是,中国在2004年之前的论文数量规模较小,但之后总体呈现显著增长态势(2010年和2015年略有起伏),并在2012年超越美国之后,成为本领域论文数量最大的国家,展现出强劲的发展势能。
为衡量近五年(2012年至2017年9月25日)论文数量最多的前10个国家在沉积地球化学方向的研究实力,进一步对论文的综合表现进行了分析(表 5)。中国作为论文总数最多的国家,在总被引用次数、被引频次≥10的论文数方面均逊于论文总数第二位的美国,但相对于其他国家则具有一定的优势。在篇均被引频次方面,中国(5.74次)与加拿大(13.14次)、法国(10.26次)、英国(9.60)、美国(9.57次)、澳大利亚(9.07次)、德国(7.55次)和意大利(6.98次)有较大差距,仅超越了印度(5.22次)和巴西(4.18次)。此外,中国在“被引频次≥30的论文”数量方面与美国的差距也比较明显,与法国、加拿大和英国比较接近,与其他国家相比则有一定的优势。总体而言,中国在“发文量”、“总被引次数”和高被引论文数这些指标上已呈现比较明显的优势,但在“篇均被引频次”这个指标上与发达国家相比仍存在较大的差距。
在研究方法方面(图 4A),国际上对元素和同位素等地球化学指标有广泛的使用。碳、氧、氢、硫元素在沉积水体及成岩流体里分布较广,其同位素分析方法成熟、测试费用经济、适用对象分布普遍,因此在沉积地球化学研究中应用极其广泛。稀土元素和微量金属也是常用的地球化学研究方法,大量应用于物源分析、沉积环境重建及成岩流体示踪等研究。有机碳、氮、磷等组分与生物生产力密切相关,是研究海洋环境特征、有机质富集及烃源岩成因等内容的必要手段。随着相关分析仪器(如LA-ICP-MS)的进步,U-Pb同位素作为同位素年代学研究的核心方法,近年来得到大量的应用。Sr-Nd同位素可以示踪沉积水体或成岩流体特征,也是化学地层研究的传统方法,因此保持较高的关注度。沉积型铀矿是战略性矿产资源,因此是重要的研究对象。此外,元素铀和钼(及同位素)可以有效约束沉积水体氧化还原状态,日益成为古海洋环境研究的常用手段。值得注意的是,砷及氟(化物)是常见的毒害元素,国际上对它们的地球化学循环过程及沉积响应也保持较高关注度。中国在研究方法上也普遍使用碳、氧、氢、硫等同位素,以及常有的稀土元素和微量金属元素,并对生物生产力相关的化学组分(如有机碳、氮、磷等)开展了大量工作(图 4B)。同时,U-Pb同位素方法在中国沉积地球化学研究中同样占有十分重要的地位。此外,中国对毒害元素砷也有一定量的研究,但是对氟化物的关注较少。
在研究对象方面(图 4C),国际上沉积地球化学研究主要围绕各类沉积物、沉积岩(如碳酸盐岩、白云岩、黑色页岩、砂岩),以及地下水、煤和土壤等开展工作,充分体现了沉积地球化学是地球表层物质循环及沉积响应的重要研究手段。同时,硅藻、黏土矿物、重矿物、碎屑锆石等特定沉积物是揭示物源、风化作用、海洋环境等内容的重要载体,因此也是主要的研究对象。此外,烃源岩、甲烷、页岩气、条带状铁建造等研究对象也占有很大份额,表明沉积地球化学积极面向能源和矿产勘探开发需求,发挥重要作用。中国的研究对象类别与国际相似(图 4D),但在碳酸盐岩、硅藻、甲烷、地下水、土壤等方面热度相对较弱。中国近五年对湖泊沉积物、黏土矿物、砂岩、白云岩、碎屑锆石、和页岩气等方面的研究热情增长显著。其中,国际和中国关于碎屑锆石和页岩气的研究均呈现快速增长特征,前者可能响应了技术(如LA-ICP-MS)的推广,后者可能响应了目前能源勘探开发新趋势。
在研究时代方面(图 4E),国际上沉积地球化学研究涉及新元古代、古元古代、元古宙、太古宙等前寒武纪时代较多。这一方面体现了前寒武纪时间跨度大、地质事件丰富、相关研究成果较多的特点,另一方面可能反映了前寒武纪相关时代贡献了较多的沉积物质(如碎屑锆石等)。同时,国际上对全新世、中新世、始新世等新生代关注密切,体现了沉积地球化学将今论古的特点以及对近现代全球气候变化的关注。此外,国际上对白垩纪、二叠纪、三叠纪、侏罗纪、寒武纪、奥陶纪、石炭纪、志留纪等时代也具有较高的热度,可能响应了相关时代具有重大地质环境—生物演化事件、以及发育富有机质烃源岩等特征。中国对于上述时代(尤其是新元古代、古元古代、白垩纪、二叠纪、三叠纪、寒武纪、奥陶纪等)同样保持较高热度,并且近5年总体呈现快速增长特征,体现了中国沉积学研究传统及地层分布特色(图 4F)。
在研究内容方面(图 4G),国际上对沉积物源的研究热度最高,与研究对象里的砂岩和碎屑锆石、研究方法里的U-Pb同位素等高频热词相呼应。成岩作用和风化作用是影响沉积岩化学特征的重要过程,也得到较高关注度。同时,古气候(及气候变化)、古环境(沉积环境及环境变化)、古海洋(古生产力、氧化还原环境、缺氧)等内容是沉积地球化学的传统研究主题,因此历来保持着较高的研究热度。地层年代格架及区域对比是沉积学研究的基础,因此随着同位素地层学和年代学方法的发展,地质年代及化学地层研究呈现较高热度。另一方面,热成熟度、生烃潜力、有机相等研究内容一定程度上服务于烃源岩评价工作,响应了油气勘探开发这一重大应用需求。此外,反硝化作用是重要的生物地球化学过程,与海洋氮循环及生物生产力密切相关,也呼应了古海洋这一研究热点。大氧化事件对地球表层环境及生物演化产生了深刻影响,在近5年里逐渐产出了较多的成果。与国际研究趋势相比,中国的研究内容同样集中于物源分析、构造背景、地质年代、古气候、古环境、古海洋和成岩作用等方面(图 4H)。然而,中国近年在热成熟度、生烃潜力、有机相等研究内容的增速较快,体现了我国沉积地球化学对国家油气资源需求及相关勘探开发工作的积极支持与响应。
2 中国沉积地球化学发展机遇与展望目前,中国已装备了一些国际一流的地球化学分析测试仪器,为沉积地球化学的发展奠定了较好的公共分析测试平台。中国沉积学研究者也积极应用各类地球化学手段与方法,丰富了研究内容、增强了研究能力、拓展了研究深度和广度。近年来,中国沉积地球化学快速发展,在SCI论文总量方面已具有明显优势。然而,目前中国沉积地球化学研究“拿来主义”色彩明显,在技术方法和理论方面重应用、少机理,重定性、少定量,处于被引领的状态。同时,中国沉积地球化学在研究的技术路线或目标问题方面偏于点、偶有线、少见面,缺乏大时空尺度的综合性工作。尽管与国际领先水平存在一定的差距,中国沉积地球化学基于国际发展趋势和研究动态,加强分析测试平台建设,在关键领域优化并创新方法、技术与理论,结合本国地质特色与既有优势,深化沉积地球化学在重大地质时期地球环境与生物演化、沉积矿产与化石能源形成与演化等重大科学问题上的综合性研究,有望实现引领式发展。
2.1 加强对沉积地球化学技术方法与理论的优化和创新重视对新技术方法与理论的开发。工欲善其事,必先利其器。地球化学分析测试技术,及方法理论飞速发展,为沉积学研究注入了新的活力。例如,LA-ICP-MS测试技术为碎屑锆石U-Pb年代学研究创造了条件,极大的促进了物源分析和地质年代学等研究成果的产出。
目前,国际上推出了一系列新的地球化学方法或指标,得到了越来越多的应用和重视,并展示了独特的示踪能力和应用前景。例如,运用238U/235U、δ33S、δ53Cr、δ98Mo、δ142Ce等同位素指标评估大气和海洋氧化状态[96, 228-229],运用δ18O重建地理海拔高度[71, 230],运用δ47(Clumped isotope,团簇同位素)约束沉积水体与成岩流体温度[231],运用δ26Mg、δ44Ca、δ7Li同位素揭示大陆风化作用[102, 113],运用δ66Zn同位素约束火山活动或海洋生命营养(元素)循环[142],运用δ11B同位素重建海水pH值和大气pCO2[159],运用各类生物标志物示踪生物来源及沉积环境(表 4)等。同时,一些其他新同位素(如88Sr/86Sr、δ56Fe、δ60Ni、δ137Ba、δ17O、δ205Tl、δ65Cu、δ114Cd、δ74Ge、δ202Hg、δ82Se)等也为理解沉积岩(物)形成过程与环境背景提供了新的约束手段或方法(表 3)。此外,随着单体分子分离技术的改进及质谱灵敏度的提高,单体同位素分析测试方法及技术将为精细理解和示踪沉积物质的循环与转化提供有力的工具。例如,单体分子放射性碳同位素分析(CSRA)将为解释有机碳的来源、迁移和转化等提供新的手段,具有广阔发展潜力。然而,这些新方法与新指标的相关理论基础、实验室校验及应用条件等方面仍存在不确定性,有待进一步探索和完善,是沉积地球化学研究的重要内容。值得注意的是,中国及华人学者已成功将多类新同位素方法应用于古气候、古海洋、古环境等研究中[96-97, 102, 112, 114, 130, 142, 158, 232-233],并在国际上具有较大的影响力[73, 101, 106, 234],这将为中国沉积地球化学的进一步发展提供有利条件和重要推力。
重视对传统地球化学方法的改进和优化。传统地球化学方法具有适用范围广、测试成本低等特点,对其进行优化和发展,将为沉积地球化学研究提供有力的工具。例如,铁组分分析流程及相关指标的建立[235-236],提升了对古海洋化学环境的辨识度(如缺氧铁化和硫化的识别),丰富了我们对地质历史时期(如元古宙及显生宙重大地质事件时期)海洋化学环境及生物演化的认识[13, 237-239]。碳酸盐岩的微量晶格硫酸盐(CAS)[240-241]及稀土元素[53]分析提取技术的优化,为我们获取真实、可靠的地球化学信号提供了必要的技术支持。碳酸盐岩成岩作用信号的辨识与提取,也为碳同位素化学地层对比及古海洋环境解释提供了新的制约[242-243]。另一方面,生物营养元素(N、P、Ba、Zn、Cu、Ni、Cd等)的生物地球化学循环,氧化还原敏感元素(Mn、Mo、U、V、Cr、Se等)的沉积地球化学过程,以及风化作用下元素迁移活性(如K、Na、Ca相对于Ti、Al、Sc等)和代用指标(如CIA指数、Ti/Al、K/Al比值)等研究,仍然有待深化。近年来,生物成因碳酸盐岩(如有孔虫、有壳类生物等钙质骨骼)的元素比值(如B/Ca、Ba/Ca,Mg/Ca,Sr/Ca,Mn/Ca、Na/Ca)在海洋化学组分重建及古环境解释等方面开展了一些探索,具有广阔的应用潜力[34, 244],但在适用生物类型的多样性方面仍需进一步开发,在元素富集机理及环境参数定量重建等方面仍需开展大量工作。
因此,围绕沉积物源分析(物质来源和时代、构造背景)、气候背景(大气组分和含量、大陆风化类型及强度)和海洋环境特征(氧化还原性质、温度、pH值、盐度、生物生产力)等关键参数,开发经济、高效和普适的地球化学方法及代用指标,是沉积地球化学研究者的不懈追求。加强对现代地球表层沉积物(海洋、河流、湖泊等)地球化学及沉积作用的研究,有利于建立新的理论与方法,并有助于将今论古。我国沉积地球化学可以地球关键带研究为依托,围绕土壤过程及其生物地球化学(C、N、P等)循环,研究关键带物质组分和元素在各界面迁移和转化的物理、化学和生物过程及耦合机制,建立沉积地球化学对地表过程和环境变化的响应机制及其反馈。另一方面,围绕“海洋微生物与生物地球化学循环”、“生物地球化学循环与气候变化”、“海洋物理—生物地球化学过程的相互作用”等方向开展相关研究,建立沉积地球化学对海洋过程生物作用的响应及反馈。在此基础上,依托我国完整的海相沉积记录,开展深时,尤其是关键地质时期地球表生系统环境过程—生命演化的研究,逐步实现在理论、方法和技术上的创新和引领。
2.2 加强对原位微区分析技术的开发和应用沉积岩(物)是多阶段(风化、搬运、沉积和成岩过程)和多来源(陆源碎屑、生物质、水柱自生矿物及成岩矿物)物质的组合,不同组构和组分具有不同的地质意义。因此,重视对沉积地球化学信息的精细分析。基于沉积岩(物)原生物理结构和构造特征,对比分析各组构的地球化学组成,有助于精确示踪风化(及物源)、沉积和成岩作用等地质信息。
传统上,牙钻和微钻等机械性原位微区取样是微区地球化学分析的重要预处理手段。例如,通过微区取样仪(Micromill Sampling)及配套的阴极发光(CL)显微镜(包括摄像系统),可对矿物生长环带进行识别和微区取样[245]。另一方面,近年来随着分析技术的进步,原位、微区、微量、高精度地球化学分析得到显著发展,将深刻影响沉积地球化学研究。这些技术相对于传统化学分析方法更加省时、省力、省样,具有明显优势。同时,这些技术可获取更为精细的地球化学信息,可拓展对沉积及成岩过程的认知,具有广阔的应用前景和机遇。例如,通过LA-ICP-MS、(Nano)SIMS、LCM-Raman、Micro-XRF、FE-EPMA、FE-SEM+EDS等(表 1)分析仪器,可获取高空间分辨元素或同位素分布图像,有助于重建沉积矿物及微组构的化学组分特征及时空演化[246-249],并精细示踪沉积物—微生物、或者水—岩相互作用等沉积—成岩过程[250-254]。中国已拥有多种大型原位微区分析仪器,并在沉积地球化学方面有所应用。目前,基于LA-ICP-MS的碎屑锆石U-Pb年代学工作开展最为广泛,在物源分析及地质年代学等研究方面取得了丰硕的成果。此外,中国科学院地质与地球物理研究所已利用Cameca IMS-1280型二次离子质谱仪(SIMS)开展单粒莓球状黄铁矿高精度硫同位素[255]及牙形刺原位微区氧同位素分析[256],利用LA-ICP-MS进行原位微区Sr-Nd同位素分析等工作。然而由于多种因素(如机时紧张,缺乏合适标样等),中国沉积地球化学研究对这些技术的应用十分有限。
因此,为改善测试平台方面机时少、费用高、适用对象有限等困境,中国应进一步加强沉积地球化学专业实验室或平台的建设,为沉积地球化学的技术方法创新和广泛应用提供坚实的支持。同时,为改善测试技术方面分析方法有限、适用对象严格等困境,中国可以进一步结合沉积地球化学的关键技术指标(表 2,3,4)和研究热点(图 4),优化改进已有技术方法,并加大对新技术、新方法、新标样的开发,促进相关技术的发展与推广。总之,原位微区高精度地球化学分析是未来发展趋势,中国可以建设并发挥平台优势,加强在沉积地球化学领域的应用,为学科发展注入强劲动力。
2.3 加强对沉积和成岩过程高分辨率年代学的发展与探索时间是约束地球表层系统圈层相互作用和化石能源矿产富集机制的重要参数,建立沉积与成岩过程的高分辨率年代标尺,意义重大而又充满挑战[257]。传统上,一系列同位素指标(如δ13C、δ18O、δ34S、δ15N、87Sr/86Sr等)常用于建立化学地层,并间接约束沉积地层时代。然而,海洋地球化学特征非均一性,以及后期地质过程(成岩作用、风化作用、成烃作用等)叠加改造,制约着高分辨率化学地层格架的建立。同时,化学地层里同位素指标的各种短周期非稳态波动特征的可靠识别、成因解译、绝对年龄锚定等工作是建立沉积地层高分辨率年代格架的重要事项,值得进一步开展与深化。另一方面,多种放射性同位素方法可以对沉积或成岩过程进行不同程度的年代学约束(表 3)。但是,测试周期较长、费用较高、适用材料有限、定年精度不足等因素严重制约着相关工作的开展。目前,沉积地层里的凝灰岩或砂岩的锆石U-Pb定年技术已日趋成熟,但测试精度(SIMS的年龄精度约为1%)有时仍难以满足研究需求(如事件地层学研究);因此建立单颗粒锆石ID-TIMS定年方法将有助于改善中国在相关研究中的被动地位。此外,Re-Os定年技术在黑色页岩、富有机质碳酸盐岩、或者烃类方面有所应用,但是也面临成功率较低的困境[182-187],需要进一步优化改进。另一方面,由于适合高精度定年的成岩矿物十分有限,目前对成岩过程(或事件)的年代(时间)约束难度非常大,有待突破。少数研究成功通过石英、方解石、萤石、黄铁矿等热液矿物进行了Rb-Sr [191-193]、Sm-Nd同位素[188-189]定年;类似的年代学工作可利用沉积岩缝洞或脉体充填的热液矿物进行探索,或许将改善目前的研究困局。对于碎屑岩地层,克服矿物分离、纯化难题,黏土矿物(如伊利石、海绿石)K-Ar或40Ar/39Ar法可尝试对成岩过程的年代进行一定的约束[180-181]。值得注意的是,碎屑独居石、成岩磷钇矿的U-Pb同位素定年技术进步,也为沉积地层和埋藏成岩流体事件等年代约束提供了新的手段[170-171]。因此,虽然沉积和成岩过程的年代学约束存在诸多困难,但是加强相关技术方法的改进与应用,仍有望产出高质量的成果,并对相关研究产生深刻影响。我国可以Earthtime地学计划为依托,发挥沉积地球化学(化学地层和同位素年代地层)的技术优势,综合岩石地层、生物地层和天文年代地层资料,为建立高分辨率综合年代地层格架提供关键支持。
2.4 加强对沉积地球化学数据的定量分析与模拟随着数据的积累,以及对地球化学循环过程的深入理解,目前沉积地球化学研究呈现从静态的定性—半定量描述向动态的定量分析与模型模拟的发展趋势。例如,碳—氧同位素等已广泛用于古海洋与古气候的模拟及定量重建[72-73, 258-263]。此外,通过地球化学模型计算,Mo同位素可定量评估海洋氧化程度[96],有机质或微量元素丰度等可定量评估海洋储库及古气候背景[264-265],Cr同位素可定量评估大气氧气含量[115],硫酸盐—硫化物(黄铁矿)的硫同位素体系可定量重建海洋硫酸盐浓度[266]等。另一方面,定量分析与模拟方法在早期成岩阶段有机质演化[267-268]、元素循环、甲烷迁移等研究中发挥着重要作用[269-272]。
通过建立地球化学模型对数据进行定量分析,将深化我们对地球表生圈层相互作用及其地球化学响应的认识。然而,元素地球化学循环过程、数学模型改进、边界条件优化等方面仍存在大量发展机遇,值得中国相关学者的关注。同时,我国沉积地球化学可以依托深时“元素生物地球化学循环与地球系统”、“海洋化学环境变化机理及其在气候系统中的作用”等重大关键科学问题,推进地球化学数据的定量分析与模拟工作,增强核心竞争能力。
2.5 加强对大时空尺度沉积地球化学分布和演化特征的重建与优化沉积岩(物)是地球表层物质循环过程、环境特征及生命活动的重要记录者。地质时期大气—海洋环境的地球化学参数大时空尺度的分布与演化趋势,可能响应了冰期—间冰期旋回、低频海平面变化、板块裂解聚合、造山运动、海底热液活动、火山活动、生物变革等长周期地质过程的演化。同时,大时空演化背景下,沉积地球化学异常分布特征可能记录了短周期或突发性地质过程,值得探索。因此,通过沉积地球化学对地球表层环境参数进行长时间尺度、大空间范围的重建,揭示其分布和演化趋势,具有重要的科学意义。
近年来,研究者通过长时间尺度(如宙、代、纪等)里的各类沉积岩或矿物重建了多种地球化学指标的分布特征或演化曲线。例如,地质历史时期碳酸盐岩(及磷酸盐矿物)的87Sr/86Sr、δ13C、δ18O曲线[273-275]、Zn/Fe值[276],黑色页岩的Mo [277]、V [278]、U [279]、Cr [264]等含量、及Se含量和δ82/78Se[280],条带状铁建造(BIF)的Ni/Fe值[281]、P/Fe值[282]、Ce异常和Eu异常[56]、U含量[31],硅质岩的δ30Si[84],沉积型黄铁矿的微量元素[248-249, 283-284],以及复合沉积岩或矿物的δ15N[82, 285]、δ56Fe[286]、Δ33S[287]等分布特征。此外,显生宙海水的δ34S和SO42-浓度[266]、δ88/86Sr[288]、δ44/40Ca[289]、Mg/Ca值[290],新生代海水δ18O和δ13C[58]、δ7Li[112-113]、187Os/186Os[291]等地球化学组分的分布演化特征也已得到不同程度的刻画。同时,大气O2和CO2演化曲线也已有了框架性约束[292-293]。这些研究一定程度上为认识沉积地球化学历史演化提供了重要的参考基准线,可为各时代对比研究提供重要的借鉴,同时也有助于理解地球大气—海洋环境的长周期演化及相关地质过程的沉积响应,具有重要的启示。
另一方面,大空间尺度(多大洋或陆块、多盆地、多沉积相带)的沉积地球化学研究有助于识别参数的非均质性及区域变化,有助于揭示其影响因素和控制过程。由于样品可获得性,近现代海洋沉积地球化学在大空间尺度的研究工作开展得比较好,并取得诸多影响深远的成果。然而,在深时古海洋环境重建方面,相关工作同样值得大力开展。例如,多陆块样品分析刻画出了中元古代及早新元古代海洋氧化还原状态[294-295];不同沉积相带样品分析,揭示了纳米比亚地区晚埃迪卡拉纪[296]、及扬子台地早—中寒武世[238-239]海洋氧化还原结构及其与生物演化的关系。同时,不同沉积相带的地球化学对比分析,可揭示该时期海洋化学组分的空间变化,如扬子地区埃迪卡拉纪—早寒武世海洋δ13C梯度[297-299]、及硫酸盐浓度[300]非均一性等。这些工作可以更加全面的刻画沉积地球化学的空间分布与演化,值得进一步开展。
总之,大时空尺度沉积地球化学分布和演化特征的重建与优化,可对地质历史时期环境演化提供更为全面的约束,因此得到广泛的关注和引用。中国沉积地球化学研究者一方面在继承地球化学时空分布和演化模式的基础上,重视对前人研究结果的优化与扩展;另一方面查新补缺,拓展旧地球化学指标的适用沉积岩及矿物类型,并开发新的地球化学指标,建立新的分布模式和演化基线。这些工作将有助于增强中国沉积地球化学研究成果的影响力,提升高被引频次文章的数量。
2.6 加强对关键地质时期地球表层环境—生物演化的研究关键地质时期地球表层环境—生物演化关系历来备受关注。中国具有丰富的沉积—地层资料、并具备一定的高精度生物地层及年代地层等工作基础。例如,中国保存有多个重大地质历史变革期的关键地层,如华北地台中新元古界,华南新元古界至寒武系、奥陶—志留系界线、泥盆系弗拉—法门阶界线、二叠—三叠系界线、白垩纪大洋红层及缺氧事件层段等。这为开展关键地质时期环境—生物事件的沉积地球化学研究提供了得天独厚的条件。目前,我国学者在相关领域开展了大量有益的尝试和探索,取得了一些创新成果,并在国际学术界建立了一定的声誉和影响。这是中国沉积地球化学未来值得进一步重点发展的优势方向。
因此,充分发挥沉积地球化学既有优势,采取多学科综合研究途径,为解决地质环境—生物协同演化这一重大关键科学问题,做出重要贡献。我国沉积地球化学应结合沉积学、盆地构造学、古地磁学、古生物与地层学、及地球生物学等研究成果,在统一的高精度时间框架内,综合约束地史时期重大地质事件—环境演化—生物响应的发生过程和规律。
2.7 加强对沉积矿产和化石能源形成与演化的研究重视对沉积矿产形成与演化的应用。元素在沉积岩中异常富集,可形成具有经济价值的沉积(或层控)型矿床历来备受关注。沉积地球化学可以示踪元素来源、迁移及富集过程,是揭示沉积型矿产成因机制及成矿规律的重要手段。中国沉积型矿床资源类型多样(如沉积型锰矿、磷矿、重晶石矿、条带状和粒状铁建造、砂岩型铀矿、风化型铝土矿和稀土矿、海底热液喷流沉积型矿床、黑色页岩型硫化物多金属矿、蒸发沉积矿床等),分布广泛,资源储量丰富,具有重要的经济价值。例如,华南新元古界—寒武系地层序列伴生多种沉积型矿床,如大塘坡组锰矿、陡山沱组及戈仲武组磷矿、牛蹄塘组Ni-Mo-V多金属矿和重晶石矿等。围绕元素异常富集的成矿物质来源(大陆风化作用、海底热液活动、生物富集作用等)及古海洋(海平面变化、缺氧或洋流上涌)、古构造—古地理(如断控热液喷流)与古气候(信风带、干燥或潮湿)背景,建立元素富集成矿模式,具有重要科学意义及经济价值。中国沉积地球化学研究可围绕“沉积型矿床的成矿作用与地球动力学系统演变的耦合关系”、“沉积(层控)型矿产形成机制和成矿规律与古海洋、古构造与古气候演化”等方面开展工作。
加强对化石能源形成与演化的应用。沉积地球化学服务于油气资源勘探开发,是我国该学科的一个显著特点。近年来,非常规油气资源(页岩油—气和致密油—气)显示出巨大的勘探潜力,是化石能源的重要战略接替领域,也将是沉积(及有机)地球化学的一个重要战场。中国沉积地球化学应进一步加强对烃源岩发育的地质条件,以及油气生成、运聚和成藏等过程的研究,继续发挥地球化学的示踪能力。其中,在发展先进的分离测定技术,以及新的描述和表征技术前提下,“过渡型有机质”的定性与定量研究也将会是学科未来的一个新的生长点。本领域研究应结合国家能源战略,围绕“深层油气成藏条件和油气分布规律”、“深层烃源有机质生烃和油气赋存规律”、“地球系统演化与盆地中生烃物质和储层的沉积环境”等方面开展工作。这些工作具有重要的科学意义和经济价值,将为中国沉积地球化学的发展提供持久的支持。
3 建议中国沉积地球化学应加强总体规划和战略布局,以进一步增强在国际学术界的影响力。因此,建议推进下列工作:
(1) 实验室建设方面,统筹创建以沉积地球化学为主旨的综合性实验室,建立具有国际领先优势的分析测试技术平台,为改进或创新沉积岩(物)地球化学的分析技术与理论方法提供坚实的支持。
(2) 人才培养方面,建立沉积地球化学暑期学校,组织编写专业相关教材,加强国内外人员的交流与学习,促进青年人才成长和领军人物的涌现,逐步形成创新人才团队。
(3) 数据库建设方面,适时组织相关专业人才,规划与启动中国沉积地球化学数据库建设,占据学科研究战略高地。
4 结语沉积地球化学是沉积学的重要研究内容和手段,在认识地球表层圈层演化和资源环境效应方面发挥着不可替代的作用。随着国家科研经费投入的增加,以及地球化学分析测试平台建设与技术进步,中国沉积地球化学迎来了快速发展期,国际学术影响力逐渐增强,但在技术方法与理论创新、及高质量成果产出等方面,仍有待改善。为提升中国沉积地球化学的研究水平和国际地位,并实现其跨越式发展,建议加强统筹规划和战略布局,以重大科学问题为导向,推进基础和前沿研究。因此建议:1)发展与创新理论、方法和技术,探索示踪物源、古气候、古环境、古生态的定量化指标,提升沉积—成岩过程年代的约束能力,重视对地球化学数据的精细提取和模型定量分析;2)加强沉积地球化学在大时空尺度的应用与探索,深化对关键地质时期地球环境—生物协同演化的研究;3)以国家经济发展需求为导向,积极发挥沉积地球化学在沉积矿产和化石能源的形成与演化研究中的重要作用。同时,鼓励运用沉积地球化学理论与方法,开展与环境科学、生命科学以及地球科学其他学科的交叉研究。
致谢: 中国沉积地球化学发展战略为王成善院士主持的国家自然科学基金重点项目“沉积学与古环境发展战略研究”中的一个专题。此研究受益于沉积学发展战略系列研讨会与会专家的讨论与交流,在此表示感谢。| [1] | Tréguer P J, de La Rocha C L. The world ocean silica cycle[J]. Annual Review of Marine Science, 2013, 5: 477–501. DOI: 10.1146/annurev-marine-121211-172346 |
| [2] | Kidder D L, Tomescu I. Biogenic chert and the Ordovician silica cycle[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2016, 458: 29–38. DOI: 10.1016/j.palaeo.2015.10.013 |
| [3] | Penman D E. Silicate weathering and North Atlantic silica burial during the Paleocene-Eocene Thermal Maximum[J]. Geology, 2016, 44(9): 731–734. DOI: 10.1130/G37704.1 |
| [4] | Zhang Y Y, Pe-Piper G, Piper D J W, et al. Sediment geochemistry as a provenance indicator:Unravelling the cryptic signatures of polycyclic sources, climate change, tectonism and volcanism[J]. Sedimentology, 2014, 61(2): 383–410. DOI: 10.1111/sed.2014.61.issue-2 |
| [5] | Bertrand S, Hughen K A, Sepúlveda J, et al. Geochemistry of surface sediments from the fjords of Northern Chilean Patagonia (44-47°S):spatial variability and implications for paleoclimate reconstructions[J]. Geochimica et Cosmochimica Acta, 2012, 76: 125–146. DOI: 10.1016/j.gca.2011.10.028 |
| [6] | Goldberg K, Humayun M. The applicability of the Chemical Index of Alteration as a paleoclimatic indicator:An example from the Permian of the Paraná Basin, Brazil[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2010, 293(1/2): 175–183. |
| [7] | Yan D T, Chen D Z, Wang Q C, et al. Large-scale climatic fluctuations in the latest Ordovician on the Yangtze block, south China[J]. Geology, 2010, 38(7): 599–602. DOI: 10.1130/G30961.1 |
| [8] | Scheffler K, Buehmann D, Schwark L. Analysis of late Palaeozoic glacial to postglacial sedimentary successions in South Africa by geochemical proxies-response to climate evolution and sedimentary environment[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2006, 240(1/2): 184–203. |
| [9] | Martinez-Ruiz F, Kastner M, Gallego-Torres D, et al. Paleoclimate and paleoceanography over the past 20, 000 yr in the Mediterranean Sea Basins as indicated by sediment elemental proxies[J]. Quaternary Science Reviews, 2015, 107: 25–46. DOI: 10.1016/j.quascirev.2014.09.018 |
| [10] | Calvert S E, Pedersen T F. Chapter fourteen:elemental proxies for palaeoclimatic and palaeoceanographic variability in marine sediments:interpretation and application[M]//CLAUDE H M, ANNE DE V. Developments in Marine Geology. Amsterdam:Elsevier, 2007:567-644. |
| [11] | Yang S Y, Jung H S, Lim D I, et al. A review on the provenance discrimination of sediments in the Yellow Sea[J]. Earth-Science Reviews, 2003, 63(1/2): 93–120. |
| [12] | Raiswell R, Canfield D E. The iron biogeochemical cycle past and present[J]. Geochemical Perspectives, 2012, 1(1): 1–220. DOI: 10.7185/geochempersp.1.1 |
| [13] | Poulton S W, Canfield D E. Ferruginous conditions:a dominant feature of the ocean through Earth's history[J]. Elements, 2011, 7(2): 107–112. DOI: 10.2113/gselements.7.2.107 |
| [14] | Johnson J E, Webb S M, Ma C, et al. Manganese mineralogy and diagenesis in the sedimentary rock record[J]. Geochimica et Cosmochimica Acta, 2016, 173: 210–231. DOI: 10.1016/j.gca.2015.10.027 |
| [15] | Maynard J B. The chemistry of manganese ores through time:a signal of increasing diversity of earth-surface environments[J]. Economic Geology, 2010, 105(3): 535–552. DOI: 10.2113/gsecongeo.105.3.535 |
| [16] | Koschinsky A, Hein J R. Marine ferromanganese encrustations:archives of changing oceans[J]. Elements, 2017, 13(3): 177–182. DOI: 10.2113/gselements.13.3.177 |
| [17] | Montagna P, Mcculloch M, Taviani M, et al. Phosphorus in cold-water corals as a proxy for seawater nutrient chemistry[J]. Science, 2006, 312(5781): 1788–1791. DOI: 10.1126/science.1125781 |
| [18] | Canfield D E, Kristensen E, Thamdrup B. The phosphorus cycle[J]. Advances in Marine Biology, 2005, 48: 419–440. DOI: 10.1016/S0065-2881(05)48011-6 |
| [19] | Slomp C P, Thomson J, De Lange G J. Controls on phosphorus regeneration and burial during formation of eastern Mediterranean sapropels[J]. Marine Geology, 2004, 203(1/2): 141–159. |
| [20] | Paytan A, Mclaughlin K. The oceanic phosphorus cycle[J]. Chemical Reviews, 2007, 107(2): 563–576. DOI: 10.1021/cr0503613 |
| [21] | Schoepfer S D, Shen J, Wei H, et al. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity[J]. Earth-Science Reviews, 2015, 149: 23–52. DOI: 10.1016/j.earscirev.2014.08.017 |
| [22] | Tyson R V. The "productivity versus preservation" controversy:cause, flaws, and resolution[M]//HARRIS N B. The Deposition of Organic-Carbon-Rich Sediments:Models, Mechanisms, and Consequences. Provo, UT, USA:SEPM, 2005, 82:17-33. |
| [23] | Zhang S C, Wang X M, Wang H J, et al. The oxic degradation of sedimentary organic matter 1400 Ma constrains atmospheric oxygen levels[J]. Biogeosciences, 2017, 14(8): 2133–2149. DOI: 10.5194/bg-14-2133-2017 |
| [24] | Singh P. Major, trace and REE geochemistry of the Ganga River sediments:influence of provenance and sedimentary processes[J]. Chemical Geology, 2009, 266(3/4): 242–255. |
| [25] | Shigemitsu M, Narita H, Watanabe Y W, et al. Ba, Si, U, Al, Sc, La, Th, C and 13C/12C in a sediment core in the western subarctic Pacific as proxies of past biological production[J]. Marine Chemistry, 2007, 106(3/4): 442–455. |
| [26] | Robbins L J, Lalonde S V, Planavsky N J, et al. Trace elements at the intersection of marine biological and geochemical evolution[J]. Earth-Science Reviews, 2016, 163: 323–348. DOI: 10.1016/j.earscirev.2016.10.013 |
| [27] | Algeo T J, Rowe H. Paleoceanographic applications of trace-metal concentration data[J]. Chemical Geology, 2012, 324-325: 6–18. DOI: 10.1016/j.chemgeo.2011.09.002 |
| [28] | Tribovillard N, Algeo T J, Lyons T, et al. Trace metals as paleoredox and paleoproductivity proxies:an update[J]. Chemical Geology, 2006, 232(1/2): 12–32. |
| [29] | Little S H, Vance D, Lyons T W, et al. Controls on trace metal authigenic enrichment in reducing sediments:Insights from modern oxygen-deficient settings[J]. American Journal of Science, 2015, 315(2): 77–119. DOI: 10.2475/02.2015.01 |
| [30] | Algeo T J, Tribovillard N. Environmental analysis of paleoceanographic systems based on molybdenum-uranium covariation[J]. Chemical Geology, 2009, 268(3/4): 211–225. |
| [31] | Partin C A, Lalonde S V, Planavsky N J, et al. Uranium in iron formations and the rise of atmospheric oxygen[J]. Chemical Geology, 2013, 362: 82–90. DOI: 10.1016/j.chemgeo.2013.09.005 |
| [32] | Tribovillard N, Algeo T J, Baudin F, et al. Analysis of marine environmental conditions based onmolybdenum-uranium covariation-applications to Mesozoic paleoceanography[J]. Chemical Geology, 2012, 324-325: 46–58. DOI: 10.1016/j.chemgeo.2011.09.009 |
| [33] | Keul N, Langer G, Thoms S, et al. Exploring foraminiferal Sr/Ca as a new carbonate system proxy[J]. Geochimica et Cosmochimica Acta, 2017, 202: 374–386. DOI: 10.1016/j.gca.2016.11.022 |
| [34] | Quintana Krupinski N B, Russell A D, Pak D K, et al. Core-top calibration of B/Ca in Pacific Ocean Neogloboquadrinaincompta and Globigerinabulloides as a surface water carbonate system proxy[J]. Earth and Planetary Science Letters, 2017, 466: 139–151. DOI: 10.1016/j.epsl.2017.03.007 |
| [35] | Hardisty D S, Lu Z L, Bekker A, et al. Perspectives on Proterozoic surface ocean redox from iodine contents in ancient and recent carbonate[J]. Earth and Planetary Science Letters, 2017, 463: 159–170. DOI: 10.1016/j.epsl.2017.01.032 |
| [36] | Hardisty D S, Lu Z L, Planavsky N J, et al. An iodine record of Paleoproterozoic surface ocean oxygenation[J]. Geology, 2014, 42(7): 619–622. DOI: 10.1130/G35439.1 |
| [37] | Lu Z L, Jenkyns H C, Rickaby R E M. Iodine to calcium ratios in marine carbonate as a paleo-redox proxy during oceanic anoxic events[J]. Geology, 2010, 38(12): 1107–1110. DOI: 10.1130/G31145.1 |
| [38] | Zhou X L, Jenkyns H C, Owens J D, et al. Upper ocean oxygenation dynamics from I/Ca ratios during the Cenomanian-Turonian OAE 2[J]. Paleoceanography, 2015, 30(5): 510–526. DOI: 10.1002/2014PA002741 |
| [39] | Griffith E M, Paytan A. Barite in the ocean-occurrence, geochemistry and palaeoceanographic applications[J]. Sedimentology, 2012, 59(6): 1817–1835. DOI: 10.1111/sed.2012.59.issue-6 |
| [40] | Paytan A, Griffith E M. Marine barite:recorder of variations in ocean export productivity[J]. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 2007, 54(5/6/7): 687–705. |
| [41] | Rehkämper M, Nielsen S G. The mass balance of dissolved thallium in the oceans[J]. Marine Chemistry, 2004, 85(3/4): 125–139. |
| [42] | Tokunaga K, Uruga T, Nitta K, et al. Application of arsenic in barite as a redox indicator for suboxic/anoxic redox condition[J]. Chemical Geology, 2016, 447: 59–69. DOI: 10.1016/j.chemgeo.2016.10.016 |
| [43] | Vandenbroucke T R, Emsbo P, Munnecke A, et al. Metal-induced malformations in early Palaeozoic plankton are harbingers of mass extinction[J]. Nature Communications, 2015, 6: 7966. DOI: 10.1038/ncomms8966 |
| [44] | Bowell R J, Alpers C N, Jamieson H E, et al. The environmental geochemistry of arsenic-an overview[J]. Reviews in Mineralogy and Geochemistry, 2014, 79(1): 1–16. DOI: 10.2138/rmg.2014.79.1 |
| [45] | Smedley P L, Kinniburgh D G. A review of the source, behaviour and distribution of arsenic in natural waters[J]. Applied Geochemistry, 2002, 17(5): 517–568. DOI: 10.1016/S0883-2927(02)00018-5 |
| [46] | Jones D S, Martini A M, FikE D A, et al. A volcanic trigger for the Late Ordovician mass extinction? Mercury data from south China and Laurentia[J]. Geology, 2017, 45(7): 631–634. DOI: 10.1130/G38940.1 |
| [47] | Percival L M E, Ruhl M, Hesselbo S P, et al. Mercury evidence for pulsed volcanism during the end-Triassic mass extinction[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(30): 7929–7934. DOI: 10.1073/pnas.1705378114 |
| [48] | Bergquist B A. Mercury, volcanism, and mass extinctions[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(33): 8675–8677. DOI: 10.1073/pnas.1709070114 |
| [49] | Han Y, Huh Y, Derry L. Ge/Si ratios indicating hydrothermal and sulfide weathering input to rivers of the Eastern Tibetan Plateau and Mt. Baekdu[J]. Chemical Geology, 2015, 410: 40–52. DOI: 10.1016/j.chemgeo.2015.06.001 |
| [50] | Dong L, Shen B, Lee C T A, et al. Germanium/silicon of the Ediacaran-Cambrian Laobao cherts:implications for the bedded chert formation and paleoenvironment interpretations[J]. Geochemistry, Geophysics, Geosystems, 2015, 16(3): 751–763. DOI: 10.1002/2014GC005595 |
| [51] | Shen B, Lee C T A, Xiao S H. Germanium/silica ratios in diagenetic chert nodules from the Ediacaran Doushantuo Formation, South China[J]. Chemical Geology, 2011, 280(3/4): 323–335. |
| [52] | Webb G E, Kamber B S. Rare earth elements in Holocene reefal microbialites:a new shallow seawater proxy[J]. Geochimica et Cosmochimica Acta, 2000, 64(9): 1557–1565. DOI: 10.1016/S0016-7037(99)00400-7 |
| [53] | Tostevin R, Shields G A, Tarbuck G M, et al. Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings[J]. Chemical Geology, 2016, 438: 146–162. DOI: 10.1016/j.chemgeo.2016.06.027 |
| [54] | Slack J F, Grenne T, Bekker A, et al. Suboxic deep seawater in the late Paleoproterozoic:evidence from hematitic chert and iron formation related to seafloor-hydrothermal sulfide deposits, central Arizona, USA[J]. Earth and Planetary Science Letters, 2007, 255(1/2): 243–256. |
| [55] | Douville E, Bienvenu P, Charlou J L, et al. Yttrium and rare earth elements in fluids from various deep-sea hydrothermal systems[J]. Geochimica et Cosmochimica Acta, 1999, 63(5): 627–643. DOI: 10.1016/S0016-7037(99)00024-1 |
| [56] | Kato Y, Yamaguchi K E, Ohmoto H. Rare earth elements in Precambrian banded iron formations:Secular changes of Ce and Eu anomalies and evolution of atmospheric oxygen[J]. Geological Society of America, 2006, 198: 269–289. |
| [57] | Tremaine D M, Froelich P N, Wang Y. Speleothem calcite farmed insitu:modern calibration of δ18O and δ13C paleoclimate proxies in a continuously-monitored natural cave system[J]. Geochimica et Cosmochimica Acta, 2011, 75(17): 4929–4950. DOI: 10.1016/j.gca.2011.06.005 |
| [58] | Zachos J, Pagani M, Sloan L, et al. Trends, rhythms, and aberrations in global climate 65 Ma to present[J]. Science, 2001, 292(5517): 686–693. DOI: 10.1126/science.1059412 |
| [59] | Knauth L P, Kennedy M J. The late Precambrian greening of the Earth[J]. Nature, 2009, 460(7256): 728–732. |
| [60] | Grotzinger J P, Fike D A, Fischer W W. Enigmatic origin of the largest-known carbon isotope excursion in Earth's history[J]. Nature Geoscience, 2011, 4(5): 285–292. DOI: 10.1038/ngeo1138 |
| [61] | Oehlert A M, Swart P K. Interpreting carbonate and organic carbon isotope covariance in the sedimentary record[J]. Nature Communications, 2014, 5: 4672. DOI: 10.1038/ncomms5672 |
| [62] | Liu J Z, An Z S, Wang Z S, et al. Using δDn-alkane as a proxy for paleo-environmental reconstruction:a good choice to sample at the site dominated by woods[J]. Science of the Total Environment, 2017, 599-600: 554–559. DOI: 10.1016/j.scitotenv.2017.05.004 |
| [63] | Schimmelmann A, Sessions A L, Mastalerz M. Hydrogen isotopic (D/H) composition of organic matter during diagenesis and thermal maturation[J]. Annual Review of Earth and Planetary Sciences, 2006, 34: 501–533. DOI: 10.1146/annurev.earth.34.031405.125011 |
| [64] | Huang Y S, Shuman B, Wang Y, et al. Hydrogen isotope ratios of individual lipids in lake sediments as novel tracers of climatic and environmental change:a surface sediment test[J]. Journal of Paleolimnology, 2004, 31(3): 363–375. DOI: 10.1023/B:JOPL.0000021855.80535.13 |
| [65] | Xie S, Nott C J, Avsejs L A, et al. Palaeoclimate records in compound-specific δD values of a lipid biomarker in ombrotrophic peat[J]. Organic Geochemistry, 2000, 31(10): 1053–1057. DOI: 10.1016/S0146-6380(00)00116-9 |
| [66] | Dählmann A, De Lange G J. Fluid-sediment interactions at Eastern Mediterranean mud volcanoes:a stable isotope study from ODP Leg 160[J]. Earth and Planetary Science Letters, 2003, 212(3/4): 377–391. |
| [67] | Gao Y, Ibarra D E, Wang C S, et al. Mid-latitude terrestrial climate of East Asia linked to global climate in the Late Cretaceous[J]. Geology, 2015, 43(4): 287–290. DOI: 10.1130/G36427.1 |
| [68] | Sun Y D, Joachimski M M, Wignall P B, et al. Lethally hot temperatures during the Early Triassic greenhouse[J]. Science, 2012, 338(6105): 366–370. DOI: 10.1126/science.1224126 |
| [69] | Wang Y J, Cheng H, Edwards R L, et al. Millennial-and orbital-scale changes in the East Asian monsoon over the past 224, 000 years[J]. Nature, 2008, 451(7182): 1090–1093. DOI: 10.1038/nature06692 |
| [70] | Zhao Y Y, Zheng Y F. Stable isotope evidence for involvement of deglacial meltwater in Ediacaran carbonates in South China[J]. Chemical Geology, 2010, 271(1/2): 86–100. |
| [71] | Rowley D B, Garzione C N. Stable isotope-based paleoaltimetry[J]. Annual Review of Earth and Planetary Sciences, 2007, 35(1): 463–508. DOI: 10.1146/annurev.earth.35.031306.140155 |
| [72] | Bao H M, Lyons J R, Zhou C M. Triple oxygen isotope evidence for elevated CO2 levels after a Neoproterozoic glaciation[J]. Nature, 2008, 453(7194): 504–506. DOI: 10.1038/nature06959 |
| [73] | Bao H M, Cao X B, Hayles J A. Triple oxygen isotopes:fundamental relationships and applications[J]. Annual Review of Earth and Planetary Sciences, 2016, 44: 463–492. DOI: 10.1146/annurev-earth-060115-012340 |
| [74] | Peng Y B, Bao H M, Pratt L M, et al. Widespread contamination of carbonate-associated sulfate by present-day secondary atmospheric sulfate:evidence from triple oxygen isotopes[J]. Geology, 2014, 42(9): 815–818. DOI: 10.1130/G35852.1 |
| [75] | Eiler J M. Paleoclimate reconstruction using carbonate clumped isotope thermometry[J]. Quaternary Science Reviews, 2011, 30(25/26): 3575–3588. |
| [76] | Loyd S J, Corsetti F A, Eiler J M, et al. Determining the diagenetic conditions of concretion formation:assessing temperatures and pore waters using clumped isotopes[J]. Journal of Sedimentary Research, 2012, 82(12): 1006–1016. DOI: 10.2110/jsr.2012.85 |
| [77] | Dennis K J, Schrag D P. Clumped isotope thermometry of carbonatites as an indicator of diagenetic alteration[J]. Geochimica et Cosmochimica Acta, 2010, 74(14): 4110–4122. DOI: 10.1016/j.gca.2010.04.005 |
| [78] | Ono S. Photochemistry of sulfur dioxide and the origin of mass-independent isotope fractionation in earth's atmosphere[J]. Annual Review of Earth and Planetary Sciences, 2017, 45: 301–329. DOI: 10.1146/annurev-earth-060115-012324 |
| [79] | Canfield D E. Biogeochemistry of sulfur isotopes[J]. Reviews in Mineralogy and Geochemistry, 2001, 43(1): 607–636. DOI: 10.2138/gsrmg.43.1.607 |
| [80] | Canfield D E, Raiswell R. The evolution of the sulfur cycle[J]. American Journal of Science, 1999, 299(7/8/9): 697–723. |
| [81] | Fike D A, Bradley A S, Rose C V. Rethinking the ancient sulfur cycle[J]. Annual Review of Earth and Planetary Sciences, 2015, 43: 593–622. DOI: 10.1146/annurev-earth-060313-054802 |
| [82] | Stüeken E E, Kipp M A, Koehler M C, et al. The evolution of Earth's biogeochemical nitrogen cycle[J]. Earth-Science Reviews, 2016, 160: 220–239. DOI: 10.1016/j.earscirev.2016.07.007 |
| [83] | Canfield D E, Glazer A N, Falkowski P G. The evolution and future of Earth's nitrogen cycle[J]. Science, 2010, 330(6001): 192–196. DOI: 10.1126/science.1186120 |
| [84] | Robert F, Chaussidon M. A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts[J]. Nature, 2006, 443(7114): 969–972. DOI: 10.1038/nature05239 |
| [85] | Frings P J, Clymans W, Fontorbe G, et al. The continental Si cycle and its impact on the ocean Si isotope budget[J]. Chemical Geology, 2016, 425: 12–36. DOI: 10.1016/j.chemgeo.2016.01.020 |
| [86] | Poitrasson F. Silicon isotope geochemistry[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 289–344. DOI: 10.2138/rmg.2017.82.8 |
| [87] | Shields G A. A normalised seawater strontium isotope curve:possible implications for Neoproterozoic-Cambrian weathering rates and the further oxygenation of the Earth[J]. eEarth, 2007, 2(2): 35–42. DOI: 10.5194/ee-2-35-2007 |
| [88] | Davis A C, Bickle M J, Teagle D A H. Imbalance in the oceanic strontium budget[J]. Earth and Planetary Science Letters, 2003, 211(1/2): 173–187. |
| [89] | Krabbenh ft A, Eisenhauer A, B hm F, et al. Constraining the marine strontium budget with natural strontium isotope fractionations (87Sr/86Sr*, δ88/86Sr) of carbonates, hydrothermal solutions and river waters[J]. Geochimica et Cosmochimica Acta, 2010, 74(14): 4097–4109. DOI: 10.1016/j.gca.2010.04.009 |
| [90] | Pearce C R, Parkinson I J, Gaillardet J, et al. Reassessing the stable (δ88/86Sr) and radiogenic (87Sr/86Sr) strontium isotopic composition of marine inputs[J]. Geochimica et Cosmochimica Acta, 2015, 157: 125–146. DOI: 10.1016/j.gca.2015.02.029 |
| [91] | Nie J S, Horton B K, Saylor J E, et al. Integrated provenance analysis of a convergent retroarc foreland system:U-Pb ages, heavy minerals, Nd isotopes, and sandstone compositions of the Middle Magdalena Valley basin, northern Andes, Colombia[J]. Earth-Science Reviews, 2012, 110(1/2/3/4): 111–126. |
| [92] | Lacan F, Tachikawa K, Jeandel C. Neodymium isotopic composition of the oceans:a compilation of seawater data[J]. Chemical Geology, 2012, 300-301: 177–184. DOI: 10.1016/j.chemgeo.2012.01.019 |
| [93] | Grasse P, Bosse L, Hathorne E C, et al. Short-term variability of dissolved rare earth elements and neodymium isotopes in the entire water column of the Panama Basin[J]. Earth and Planetary Science Letters, 2017, 475: 242–253. DOI: 10.1016/j.epsl.2017.07.022 |
| [94] | Gordon G W, Lyons T W, Arnold G L, et al. When do black shales tell molybdenum isotope tales?[J]. Geology, 2009, 37(6): 535–538. DOI: 10.1130/G25186A.1 |
| [95] | Kendall B, Dahl T W, Anbar A D. The stable isotope geochemistry of molybdenum[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 683–732. DOI: 10.2138/rmg.2017.82.16 |
| [96] | Chen X, Ling H F, Vance D, et al. Rise to modern levels of ocean oxygenation coincided with the Cambrian radiation of animals[J]. Nature Communication, 2015, 6: 7142. DOI: 10.1038/ncomms8142 |
| [97] | Wen H J, Fan H F, Zhang Y X, et al. Reconstruction of early Cambrian ocean chemistry from Mo isotopes[J]. Geochimica et Cosmochimica Acta, 2015, 164: 1–16. DOI: 10.1016/j.gca.2015.05.008 |
| [98] | Severmann S, Mills R A, Palmer M R, et al. The role of prokaryotes in subsurface weathering of hydrothermal sediments:a combined geochemical and microbiological investigation[J]. Geochimica et Cosmochimica Acta, 2006, 70(7): 1677–1694. DOI: 10.1016/j.gca.2005.12.008 |
| [99] | Severmann S, Lyons T W, Anbar A, et al. Modern iron isotope perspective on the benthic iron shuttle and the redox evolution of ancient oceans[J]. Geology, 2008, 36(6): 487–490. DOI: 10.1130/G24670A.1 |
| [100] | Dauphas N, John S G, Rouxel O. Iron isotope systematics[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 415–510. DOI: 10.2138/rmg.2017.82.11 |
| [101] | Zhu X K, O'nions R K, Guo Y L, et al. Secular variation of iron isotopes in North Atlantic deep water[J]. Science, 2000, 287(5460): 2000–2002. DOI: 10.1126/science.287.5460.2000 |
| [102] | Huang K J, Teng F Z, Shen B, et al. Episode of intense chemical weathering during the termination of the 635 Ma Marinoan glaciation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(52): 14904–14909. DOI: 10.1073/pnas.1607712113 |
| [103] | Higgins J A, Schrag D P. The Mg isotopic composition of Cenozoic seawater-evidence for a link between Mg-clays, seawater Mg/Ca, and climate[J]. Earth and Planetary Science Letters, 2015, 416: 73–81. DOI: 10.1016/j.epsl.2015.01.003 |
| [104] | Higgins J A, Schrag D P. Constraining magnesium cycling in marine sediments using magnesium isotopes[J]. Geochimica et Cosmochimica Acta, 2010, 74(17): 5039–5053. DOI: 10.1016/j.gca.2010.05.019 |
| [105] | Tipper E T, Galy A, Gaillardet J, et al. The magnesium isotope budget of the modern ocean:constraints from riverine magnesium isotope ratios[J]. Earth and Planetary Science Letters, 2006, 250(1/2): 241–253. |
| [106] | Teng F Z. Magnesium isotope geochemistry[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 219–287. DOI: 10.2138/rmg.2017.82.7 |
| [107] | Jacobson A D, Grace Andrews M, Lehn G O, et al. Silicate versus carbonate weathering in Iceland:new insights from Ca isotopes[J]. Earth and Planetary Science Letters, 2015, 416: 132–142. DOI: 10.1016/j.epsl.2015.01.030 |
| [108] | Husson J M, Higgins J A, Maloof A C, et al. Ca and Mg isotope constraints on the origin of Earth's deepest δ13C excursion[J]. Geochimica et Cosmochimica Acta, 2015, 160: 243–266. DOI: 10.1016/j.gca.2015.03.012 |
| [109] | Fantle M S. Calcium isotopic evidence for rapid recrystallization of bulk marine carbonates and implications for geochemical proxies[J]. Geochimica et Cosmochimica Acta, 2015, 148: 378–401. DOI: 10.1016/j.gca.2014.10.005 |
| [110] | Fantle M S, Tipper E T. Calcium isotopes in the global biogeochemical Ca cycle:implications for development of a Ca isotope proxy[J]. Earth-Science Reviews, 2014, 129: 148–177. DOI: 10.1016/j.earscirev.2013.10.004 |
| [111] | Wanner C, Sonnenthal E L, Liu X M. Seawater δ7Li:a direct proxy for global CO2 consumption by continental silicate weathering?[J]. Chemical Geology, 2014, 381: 154–167. DOI: 10.1016/j.chemgeo.2014.05.005 |
| [112] | LI G J, WEST A J. Evolution of Cenozoic seawater lithium isotopes:coupling of global denudation regime and shifting seawater sinks[J]. Earth and Planetary Science Letters, 2014, 401: 284–293. DOI: 10.1016/j.epsl.2014.06.011 |
| [113] | Misra S, Froelich P N. Lithium isotope history of Cenozoic seawater:changes in silicate weathering and reverse weathering[J]. Science, 2012, 335(6070): 818–823. DOI: 10.1126/science.1214697 |
| [114] | Wang X L, Reinhard C T, Planavsky N J, et al. Sedimentary chromium isotopic compositions across the Cretaceous OAE2 at Demerara Rise Site 1258[J]. Chemical Geology, 2016, 429: 85–92. DOI: 10.1016/j.chemgeo.2016.03.006 |
| [115] | Planavsky N J, Reinhard C T, Wang X L, et al. Low mid-proterozoic atmospheric oxygen levels and the delayed rise of animals[J]. Science, 2014, 346(6209): 635–638. DOI: 10.1126/science.1258410 |
| [116] | Qin L P, Wang X L. Chromium isotope geochemistry[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 379–414. DOI: 10.2138/rmg.2017.82.10 |
| [117] | Elliott T, Steele R C J. The isotope geochemistry of Ni[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 511–542. DOI: 10.2138/rmg.2017.82.12 |
| [118] | Porter S J, Selby D, Cameron V. Characterising the nickel isotopic composition of organic-rich marine sediments[J]. Chemical Geology, 2014, 387: 12–21. DOI: 10.1016/j.chemgeo.2014.07.017 |
| [119] | Cameron V, Vance D. Heavy nickel isotope compositions in rivers and the oceans[J]. Geochimica et Cosmochimica Acta, 2014, 128: 195–211. DOI: 10.1016/j.gca.2013.12.007 |
| [120] | Gall L, Williams H M, Siebert C, et al. Nickel isotopic compositions of ferromanganese crusts and the constancy of deep ocean inputs and continental weathering effects over the Cenozoic[J]. Earth and Planetary Science Letters, 2013, 375: 148–155. DOI: 10.1016/j.epsl.2013.05.019 |
| [121] | Georg R B, West A J, Vance D, et al. Is the marine osmium isotope record a probe for CO2 release from sedimentary rocks?[J]. Earth and Planetary Science Letters, 2013, 367: 28–38. DOI: 10.1016/j.epsl.2013.02.018 |
| [122] | Georgiev S, Stein H J, Hannah J L, et al. Hot acidic Late Permian seas stifle life in record time[J]. Earth and Planetary Science Letters, 2011, 310(3/4): 389–400. |
| [123] | Du Vivier A D C, Selby D, Sageman B B, et al. Marine 187Os/188Os isotope stratigraphy reveals the interaction of volcanism and ocean circulation during Oceanic Anoxic Event 2[J]. Earth and Planetary Science Letters, 2014, 389: 23–33. DOI: 10.1016/j.epsl.2013.12.024 |
| [124] | Foster G L, Vance D. Negligible glacial-interglacial variation in continental chemical weathering rates[J]. Nature, 2006, 444(7121): 918–921. DOI: 10.1038/nature05365 |
| [125] | Komárek M, Ettler V, Chrastny V, et al. Lead isotopes in environmental sciences:a review[J]. Environment International, 2008, 34(4): 562–577. DOI: 10.1016/j.envint.2007.10.005 |
| [126] | Basak C, Martin E E, Kamenov G D. Seawater Pb isotopes extracted from cenozoic marine sediments[J]. Chemical Geology, 2011, 286(3/4): 94–108. |
| [127] | Ling H F, Jiang S Y, Frank M, et al. Differing controls over the cenozoic Pb and Nd isotope evolution of deepwater in the central North Pacific Ocean[J]. Earth and Planetary Science Letters, 2005, 232(3/4): 345–361. |
| [128] | Rolison J M, Stirling C H, Middag R, et al. Uranium stable isotope fractionation in the Black Sea:modern calibration of the 238U/235U paleo-redox proxy[J]. Geochimica et Cosmochimica Acta, 2017, 203: 69–88. DOI: 10.1016/j.gca.2016.12.014 |
| [129] | Wang X L, Planavsky N J, Reinhard C T, et al. A Cenozoic seawater redox record derived from 238U/235U in ferromanganese crusts[J]. American Journal of Science, 2016, 316(1): 64–83. DOI: 10.2475/01.2016.02 |
| [130] | Chen X M, Romaniello S J, Anbar A D. Uranium isotope fractionation induced by aqueous speciation:IMPLICATIONS for U isotopes in marine CaCO3 as a paleoredox proxy[J]. Geochimica et Cosmochimica Acta, 2017, 215: 162–172. DOI: 10.1016/j.gca.2017.08.006 |
| [131] | Hinojosa J L, Stirling C H, Reid M R, et al. Trace metal cycling and 238U/235U in New Zealand's fjords:Implications for reconstructing global paleoredox conditions in organic-rich sediments[J]. Geochimica et Cosmochimica Acta, 2016, 179: 89–109. DOI: 10.1016/j.gca.2016.02.006 |
| [132] | Andersen M B, Stirling C H, Weyer S. Uranium isotope fractionation[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 799–850. DOI: 10.2138/rmg.2017.82.19 |
| [133] | Nakada R, Takahashi Y, Tanimizu M. Cerium stable isotope ratios in ferromanganese deposits and their potential as a paleo-redox proxy[J]. Geochimica et Cosmochimica Acta, 2016, 181: 89–100. DOI: 10.1016/j.gca.2016.02.025 |
| [134] | Nakada R, Takahashi Y, Tanimizu M. Isotopic and speciation study on cerium during its solid-water distribution with implication for Ce stable isotope as a paleo-redox proxy[J]. Geochimica et Cosmochimica Acta, 2013, 103: 49–62. DOI: 10.1016/j.gca.2012.10.045 |
| [135] | Nielsen S G, Goff M, Hesselbo S P, et al. Thallium isotopes in early diagenetic pyrite-a paleoredox proxy?[J]. Geochimica et Cosmochimica Acta, 2011, 75(21): 6690–6704. DOI: 10.1016/j.gca.2011.07.047 |
| [136] | Owens J D, Nielsen S G, Horner T J, et al. Thallium-isotopic compositions of euxinic sediments as a proxy for global manganese-oxide burial[J]. Geochimica et Cosmochimica Acta, 2017, 213: 291–307. DOI: 10.1016/j.gca.2017.06.041 |
| [137] | Ostrander C M, Owens J D, Nielsen S G. Constraining the rate of oceanic deoxygenation leading up to a Cretaceous Oceanic Anoxic Event (OAE-2:~94 Ma)[J]. Science Advances, 2017, 3(8): e1701020. DOI: 10.1126/sciadv.1701020 |
| [138] | Stüeken E E. Selenium isotopes as a biogeochemical proxy in deep time[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 657–682. DOI: 10.2138/rmg.2017.82.15 |
| [139] | Mitchell K, Mansoor S Z, Mason P R D, et al. Geological evolution of the marine selenium cycle:Insights from the bulk shale δ82/76Se record and isotope mass balance modeling[J]. Earth and Planetary Science Letters, 2016, 441: 178–187. DOI: 10.1016/j.epsl.2016.02.030 |
| [140] | Kipp M A, Stüeken E E, Bekker A, et al. Selenium isotopes record extensive marine suboxia during the great oxidation event[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(5): 875–880. DOI: 10.1073/pnas.1615867114 |
| [141] | Stüeken E E, Foriel J, Buick R, et al. Selenium isotope ratios, redox changes and biological productivity across the end-Permian mass extinction[J]. Chemical Geology, 2015, 410: 28–39. DOI: 10.1016/j.chemgeo.2015.05.021 |
| [142] | Liu S A, Wu H C, Shen S Z, et al. Zinc isotope evidence for intensive magmatism immediately before the end-Permian mass extinction[J]. Geology, 2017, 45(4): 343–346. DOI: 10.1130/G38644.1 |
| [143] | K bberich M, Vance D. Kinetic control on Zn isotope signatures recorded in marine diatoms[J]. Geochimica et Cosmochimica Acta, 2017, 210: 97–113. DOI: 10.1016/j.gca.2017.04.014 |
| [144] | Kunzmann M, Halverson G P, Sossi P A, et al. Zn isotope evidence for immediate resumption of primary productivity after snowball Earth[J]. Geology, 2012, 41(1): 27–30. |
| [145] | Maréchal C N, Nicolas E, Douchet C, et al. Abundance of zinc isotopes as a marine biogeochemical tracer[J]. Geochemistry Geophysics Geosystems, 2000, 1(5): 1015. |
| [146] | Little S H, Vance D, Mcmanus J, et al. Copper isotope signatures in modern marine sediments[J]. Geochimica et Cosmochimica Acta, 2017, 212: 253–273. DOI: 10.1016/j.gca.2017.06.019 |
| [147] | Guinoiseau D, Gélabert A, Allard T, et al. Zinc and copper behaviour at the soil-river interface:new insights by Zn and Cu isotopes in the organic-rich Rio Negro basin[J]. Geochimica et Cosmochimica Acta, 2017, 213: 178–197. DOI: 10.1016/j.gca.2017.06.030 |
| [148] | Moynier F, Vance D, Fujii T, et al. The isotope geochemistry of zinc and copper[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 543–600. DOI: 10.2138/rmg.2017.82.13 |
| [149] | Horner T J, Kinsley C W, Nielsen S G. Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation[J]. Earth and Planetary Science Letters, 2015, 430: 511–522. DOI: 10.1016/j.epsl.2015.07.027 |
| [150] | Cao Z M, Siebert C, Hathorne E C, et al. Constraining the oceanic barium cycle with stable barium isotopes[J]. Earth and Planetary Science Letters, 2016, 434: 1–9. DOI: 10.1016/j.epsl.2015.11.017 |
| [151] | Grasby S E, Shen W J, Yin R S, et al. Isotopic signatures of mercury contamination in latest Permian oceans[J]. Geology, 2017, 45(1): 55–58. DOI: 10.1130/G38487.1 |
| [152] | Blum J D, Sherman L S, Johnson M W. Mercury isotopes in earth and environmental sciences[J]. Annual Review of Earth and Planetary Sciences, 2014, 42: 249–269. DOI: 10.1146/annurev-earth-050212-124107 |
| [153] | Bergquist B A, Blum J D. The odds and evens of mercury isotopes:applications of mass-dependent and mass-independent isotope fractionation[J]. Elements, 2009, 5(6): 353–357. DOI: 10.2113/gselements.5.6.353 |
| [154] | Janssen D J, Conway T M, John S G, et al. Undocumented water column sink for cadmium in open ocean oxygen-deficient zones[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(19): 6888–6893. DOI: 10.1073/pnas.1402388111 |
| [155] | Conway T M, John S G. Biogeochemical cycling of cadmium isotopes along a high-resolution section through the North Atlantic Ocean[J]. Geochimica et Cosmochimica Acta, 2015, 148: 269–283. DOI: 10.1016/j.gca.2014.09.032 |
| [156] | Guillermic M, Lalonde S V, Hendry K R, et al. The isotope composition of inorganic germanium in seawater and deep sea sponges[J]. Geochimica et Cosmochimica Acta, 2017, 212: 99–118. DOI: 10.1016/j.gca.2017.06.011 |
| [157] | Baronas J J, Hammond D E, Mcmanus J, et al. A global Ge isotope budget[J]. Geochimica et Cosmochimica Acta, 2017, 203: 265–283. DOI: 10.1016/j.gca.2017.01.008 |
| [158] | Zhang S, Henehan M J, Hull P M, et al. Investigating controls on boron isotope ratios in shallow marine carbonates[J]. Earth and Planetary Science Letters, 2017, 458: 380–393. DOI: 10.1016/j.epsl.2016.10.059 |
| [159] | Rasbury E T, Hemming N G. Boron isotopes:a "paleo-pH meter" for tracking ancient atmospheric CO2[J]. Elements, 2017, 13(4): 243–248. |
| [160] | Foster G L, Rae J W B. Reconstructing ocean pH with boron isotopes in foraminifera[J]. Annual Review of Earth and Planetary Sciences, 2016, 44: 207–237. DOI: 10.1146/annurev-earth-060115-012226 |
| [161] | Clarkson M O, Kasemann S A, Wood R A, et al. Ocean acidification and the Permo-Triassic mass extinction[J]. Science, 2015, 348(6231): 229–232. DOI: 10.1126/science.aaa0193 |
| [162] | Peucker-Ehrenbrink B, Ravizza G, Winckler G. Geochemical tracers of extraterrestrial matter in sediments[J]. Elements, 2016, 12(3): 191–196. DOI: 10.2113/gselements.12.3.191 |
| [163] | Resing J A, Sedwick P N, German C R, et al. Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean[J]. Nature, 2015, 523(7559): 200–203. DOI: 10.1038/nature14577 |
| [164] | Murphy B H, Farley K A, Zachos J C. An extraterrestrial 3He-based timescale for the Paleocene-Eocene thermal maximum (PETM) from Walvis Ridge, IODP Site 1266[J]. Geochimica et Cosmochimica Acta, 2010, 74(17): 5098–5108. DOI: 10.1016/j.gca.2010.03.039 |
| [165] | Hua Q. Radiocarbon:a chronological tool for the recent past[J]. Quaternary Geochronology, 2009, 4(5): 378–390. DOI: 10.1016/j.quageo.2009.03.006 |
| [166] | Lund D C, Mix A C, Southon J. Increased ventilation age of the deep northeast Pacific Ocean during the last deglaciation[J]. Nature Geoscience, 2011, 4(11): 771–774. DOI: 10.1038/ngeo1272 |
| [167] | Muscheler R, Kromer B, Bj rck S, et al. Tree rings and ice cores reveal 14C calibration uncertainties during the Younger Dryas[J]. Nature Geoscience, 2008, 1(4): 263–267. DOI: 10.1038/ngeo128 |
| [168] | Shen S Z, Crowley J L, Wang Y, et al. Calibrating the end-Permian mass extinction[J]. Science, 2011, 334(6061): 1367–1372. DOI: 10.1126/science.1213454 |
| [169] | Condon D, Zhu M Y, Bowring S, et al. U-Pb ages from the Neoproterozoic Doushantuo formation, China[J]. Science, 2005, 308(5718): 95–98. DOI: 10.1126/science.1107765 |
| [170] | Zhang Y B, Li Q L, Lan Z W, et al. Diagenetic xenotime dating to constrain the initial depositional time of the Yan-Liao Rift[J]. Precambrian Research, 2015, 271: 20–32. DOI: 10.1016/j.precamres.2015.09.024 |
| [171] | Lan Z W, Li X H, Chen Z Q, et al. Diagenetic xenotime age constraints on the Sanjiaotang Formation, Luoyu Group, southern margin of the North China Craton:implications for regional stratigraphic correlation and early evolution of eukaryotes[J]. Precambrian Research, 2014, 251: 21–32. DOI: 10.1016/j.precamres.2014.06.012 |
| [172] | Gallup C D, Cheng H, Taylor F W, et al. Direct determination of the timing of sea level change during Termination Ⅱ[J]. Science, 2002, 295(5553): 310–313. DOI: 10.1126/science.1065494 |
| [173] | Wang Y J, Cheng H, Edwards R L, et al. A high-resolution absolute-dated late Pleistocene monsoon record from Hulu Cave, China[J]. Science, 2001, 294(5550): 2345–2348. DOI: 10.1126/science.1064618 |
| [174] | Cheng H, Adkins J, Edwards R L, et al. U-Th dating of deep-sea corals[J]. Geochimica et Cosmochimica Acta, 2000, 64(14): 2401–2416. DOI: 10.1016/S0016-7037(99)00422-6 |
| [175] | Chew D M, Spikings R A. Geochronology and thermochronology using apatite:time and temperature, lower crust to surface[J]. Elements, 2015, 11(3): 189–194. DOI: 10.2113/gselements.11.3.189 |
| [176] | Chang J, Qiu N S, Li J W. Tectono-thermal evolution of the northwestern edge of the Tarim Basin in China:constraints from apatite (U-Th)/He thermochronology[J]. Journal of Asian Earth Sciences, 2012, 61: 187–198. DOI: 10.1016/j.jseaes.2012.09.020 |
| [177] | Qiu N S, Chang J, Zuo Y H, et al. Thermal evolution and maturation of lower Paleozoic source rocks in the Tarim Basin, northwest China[J]. AAPG Bulletin, 2012, 96(5): 789–821. DOI: 10.1306/09071111029 |
| [178] | Farley K A. (U-Th)/He dating:techniques, calibrations, and applications[J]. Reviews in Mineralogy and Geochemistry, 2002, 47(1): 819–844. DOI: 10.2138/rmg.2002.47.18 |
| [179] | Jourdan F, Hodges K, Sell B, et al. High-precision dating of the Kalkarindji large igneous province, Australia, and synchrony with the Early-Middle Cambrian (Stage 4-5) extinction[J]. Geology, 2014, 42(6): 543–546. DOI: 10.1130/G35434.1 |
| [180] | Clauer N. The K-Ar and 40Ar/39Ar methods revisited for dating fine-grained K-bearing clay minerals[J]. Chemical Geology, 2013, 354: 163–185. DOI: 10.1016/j.chemgeo.2013.05.030 |
| [181] | Clauer N, Zwingmann H, Liewig N, et al. Comparative 40Ar/39Ar and K-Ar dating of illite-type clay minerals:a tentative explanation for age identities and differences[J]. Earth-Science Reviews, 2012, 115(1/2): 76–96. |
| [182] | Kendall B, Creaser R A, Selby D. 187Re-187Os geochronology of Precambrian organic-rich sedimentary rocks[J]. Geological Society, London, Special Publications, 2009, 326(1): 85–107. DOI: 10.1144/SP326.5 |
| [183] | Zhu B, Becker H, Jiang S Y, et al. Re-Os geochronology of black shales from the Neoproterozoic Doushantuo Formation, Yangtze platform, South China[J]. Precambrian Research, 2013, 225: 67–76. DOI: 10.1016/j.precamres.2012.02.002 |
| [184] | Zhao H, Li C, Jiang X J, et al. Direct radiometric dating of Limestone from Changxing Permian-Triassic boundary using the Re-Os geochronometer[J]. Chinese Science Bulletin, 2015, 60(23): 2209–2215. DOI: 10.1360/N972015-00409 |
| [185] | Xu L G, Lehmann B, Mao J W, et al. Re-Os age of polymetallic Ni-Mo-PGE-Au mineralization in early cambrian black shales of South China-a reassessment[J]. Economic Geology, 2011, 106(3): 511–522. DOI: 10.2113/econgeo.106.3.511 |
| [186] | Selby D, Mutterlose J, Condon D J. U-Pb and Re-Os geochronology of the Aptian/Albian and Cenomanian/Turonian stage boundaries:implications for timescale calibration, osmium isotope seawater composition and Re-Os systematics in organic-rich sediments[J]. Chemical Geology, 2009, 265(3/4): 394–409. |
| [187] | Selby D, Creaser R A. Direct radiometric dating of hydrocarbon deposits using rhenium-osmium isotopes[J]. Science, 2005, 308(5726): 1293–1295. DOI: 10.1126/science.1111081 |
| [188] | Henjes-Kunst F, Prochaska W, Niedermayr A, et al. Sm-Nd dating of hydrothermal carbonate formation:an example from the Breitenau magnesite deposit (Styria, Austria)[J]. Chemical Geology, 2014, 387: 184–201. DOI: 10.1016/j.chemgeo.2014.07.025 |
| [189] | Su W C, Hu R Z, Xia B, et al. Calcite Sm-Nd isochron age of the Shuiyindong Carlin-type gold deposit, Guizhou, China[J]. Chemical Geology, 2009, 258(3/4): 269–274. |
| [190] | Barker S L L, Bennett V C, Cox S F, et al. Sm-Nd, Sr, C and O isotope systematics in hydrothermal calcite-fluorite veins:implications for fluid-rock reaction and geochronology[J]. Chemical Geology, 2009, 268(1/2): 58–66. |
| [191] | Middleton A W, Uysal I T, Bryan S E, et al. Integrating 40Ar-39Ar, 87Rb-87Sr and 147Sm-143Nd geochronology of authigenic illite to evaluate tectonic reactivation in an intraplate setting, central Australia[J]. Geochimica et Cosmochimica Acta, 2014, 134: 155–174. DOI: 10.1016/j.gca.2014.02.048 |
| [192] | Li Q L, Chen F K, Yang J H, et al. Single grain pyrite Rb-Sr dating of the Linglong gold deposit, eastern China[J]. Ore Geology Reviews, 2008, 34(3): 263–270. DOI: 10.1016/j.oregeorev.2007.10.003 |
| [193] | Yang J H, Zhou X H. Rb-Sr, Sm-Nd, and Pb isotope systematics of pyrite:implications for the age and genesis of lode gold deposits[J]. Geology, 2001, 29(8): 711–714. DOI: 10.1130/0091-7613(2001)029<0711:RSSNAP>2.0.CO;2 |
| [194] | Prahl F G, Mix A C, Sparrow M A. Alkenone paleothermometry:biological lessons from marine sediment records off western South America[J]. Geochimica et Cosmochimica Acta, 2006, 70(1): 101–117. DOI: 10.1016/j.gca.2005.08.023 |
| [195] | Tissot B P, Welte D H. Petroleum Formation and Occurrence[M]. 2nd ed. Berlin:Springer-Verlag, 1984. |
| [196] | Liu H, Liu W G. n-Alkane distributions and concentrations in algae, submerged plants and terrestrial plants from the Qinghai-Tibetan Plateau[J]. Organic Geochemistry, 2016, 99: 10–22. DOI: 10.1016/j.orggeochem.2016.06.003 |
| [197] | Feakins S J, Peters T, Wu M S, et al. Production of leaf wax n-alkanes across a tropical forest elevation transect[J]. Organic Geochemistry, 2016, 100: 89–100. DOI: 10.1016/j.orggeochem.2016.07.004 |
| [198] | Connan J, Bouroullec J, Dessort D, et al. The microbial input in carbonate-anhydrite facies of a sabkha palaeoenvironment from Guatemala:a molecular approach[J]. Organic Geochemistry, 1986, 10(1/2/3): 29–50. |
| [199] | Schinteie R, Brocks J J. Paleoecology of Neoproterozoic hypersaline environments:biomarker evidence for haloarchaea, methanogens, and cyanobacteria[J]. Geobiology, 2017, 15(5): 641–663. DOI: 10.1111/gbi.2017.15.issue-5 |
| [200] | Stolper D A, Love G D, Bates S, et al. Paleoecology and paleoceanography of the Athel silicilyte, Ediacaran-Cambrian boundary, Sultanate of Oman[J]. Geobiology, 2017, 15(3): 401–426. DOI: 10.1111/gbi.2017.15.issue-3 |
| [201] | Duda J P, Blumenberg M, Thiel V, et al. Geobiology of a palaeoecosystem with Ediacara-type fossils:the Shibantan member (dengying formation, South China)[J]. Precambrian Research, 2014, 255: 48–62. DOI: 10.1016/j.precamres.2014.09.012 |
| [202] | Maslen E, Grice K, Gale J D, et al. Crocetane:a potential marker of photic zone euxinia in thermally mature sediments and crude oils of Devonian age[J]. Organic Geochemistry, 2009, 40(1): 1–11. DOI: 10.1016/j.orggeochem.2008.10.005 |
| [203] | Aboglila S, Grice K, Trinajstic K, et al. The significance of 24-nor cholestanes, 4-methylsteranes and dinosteranes in oils and source-rocks from East Sirte Basin (Libya)[J]. Applied Geochemistry, 2011, 26(9/10): 1694–1705. |
| [204] | Brocks J J, Love G D, Summons R E, et al. Biomarker evidence for green and purple sulphur bacteria in a stratified Palaeoproterozoic sea[J]. Nature, 2005, 437(7060): 866–870. DOI: 10.1038/nature04068 |
| [205] | Koopmans M P, Schouten S, Kohnen M E, et al. Restricted utility of aryl isoprenoids as indicators for photic zone anoxia[J]. Geochimica et Cosmochimica Acta, 1996, 60(23): 4873–4876. DOI: 10.1016/S0016-7037(96)00303-1 |
| [206] | Melendez I, Grice K, Trinajstic K, et al. Biomarkers reveal the role of photic zone euxinia in exceptional fossil preservation:an organic geochemical perspective[J]. Geology, 2013, 41(2): 123–126. DOI: 10.1130/G33492.1 |
| [207] | Smolarek J, Marynowski L, Trela W, et al. Redox conditions and marine microbial community changes during the end-Ordovician mass extinction event[J]. Global and Planetary Change, 2017, 149: 105–122. DOI: 10.1016/j.gloplacha.2017.01.002 |
| [208] | Brocks J J, Schaeffer P. Okenane, a biomarker for purple sulfur bacteria (Chromatiaceae), and other new carotenoid derivatives from the 1640 Ma Barney Creek Formation[J]. Geochimica et Cosmochimica Acta, 2008, 72(5): 1396–1414. DOI: 10.1016/j.gca.2007.12.006 |
| [209] | Sachse V F, Delvaux D, Littke R. Petrological and geochemical investigations of potential source rocks of the central Congo Basin, Democratic Republic of Congo[J]. AAPG Bulletin, 2012, 96(2): 245–275. DOI: 10.1306/07121111028 |
| [210] | Miceli Romero A, Philp R P. Organic geochemistry of the Woodford Shale, southeastern Oklahoma:how variable can shales be?[J]. AAPG Bulletin, 2012, 96(3): 493–517. DOI: 10.1306/08101110194 |
| [211] | Kelly A E, Love G D, Zumberge J E, et al. Hydrocarbon biomarkers of Neoproterozoic to Lower Cambrian oils from eastern Siberia[J]. Organic Geochemistry, 2011, 42(6): 640–654. DOI: 10.1016/j.orggeochem.2011.03.028 |
| [212] | Korkmaz S, Kara-Gülbay R, Iztan Y H. Organic geochemistry of the Lower Cretaceous black shales and oil seep in the Sinop Basin, Northern Turkey:an oil-source rock correlation study[J]. Marine and Petroleum Geology, 2013, 43: 272–283. DOI: 10.1016/j.marpetgeo.2013.02.003 |
| [213] | Araújo B Q, De Almeida Azevedo D. Uncommon steranes in Brazilian marginal crude oils:dinoflagellate molecular fossils in the Sergipe-Alagoas Basin, Brazil[J]. Organic Geochemistry, 2016, 99: 38–52. DOI: 10.1016/j.orggeochem.2016.06.004 |
| [214] | Rohrssen M, Gill B C, Love G D. Scarcity of the C30 sterane biomarker, 24-n-propylcholestane, in Lower Paleozoic marine paleoenvironments[J]. Organic Geochemistry, 2015, 80: 1–7. DOI: 10.1016/j.orggeochem.2014.11.008 |
| [215] | Gold D A, O'reilly S S, Watson J, et al. Lipidomics of the sea sponge Amphimedon queenslandica and implication for biomarker geochemistry[J]. Geobiology, 2017, 15(6): 836–843. DOI: 10.1111/gbi.2017.15.issue-6 |
| [216] | Brocks J J, Jarrett A J M, Sirantoine E, et al. The rise of algae in Cryogenian oceans and the emergence of animals[J]. Nature, 2017, 548(7669): 578–581. DOI: 10.1038/nature23457 |
| [217] | Luo Q Y, George S C, Xu Y H, et al. Organic geochemical characteristics of the Mesoproterozoic Hongshuizhuang Formation from northern China:implications for thermal maturity and biological sources[J]. Organic Geochemistry, 2016, 99: 23–37. DOI: 10.1016/j.orggeochem.2016.05.004 |
| [218] | Eigenbrode J L, Freeman K H, Summons R E. Methylhopane biomarker hydrocarbons in Hamersley Province sediments provide evidence for Neoarchean aerobiosis[J]. Earth and Planetary Science Letters, 2008, 273(3/4): 323–331. |
| [219] | Xie S C, Algeo T J, Zhou W F, et al. Contrasting microbial community changes during mass extinctions at the Middle/Late Permian and Permian/Triassic boundaries[J]. Earth and Planetary Science Letters, 2017, 460: 180–191. DOI: 10.1016/j.epsl.2016.12.015 |
| [220] | Payne J L, Clapham M E. End-permian mass extinction in the oceans:an ancient analog for the twenty-first century?[J]. Annual Review of Earth and Planetary Sciences, 2012, 40: 89–111. DOI: 10.1146/annurev-earth-042711-105329 |
| [221] | Whiteside J H, Grice K. Biomarker records associated with mass extinction events[J]. Annual Review of Earth and Planetary Sciences, 2016, 44: 581–612. DOI: 10.1146/annurev-earth-060115-012501 |
| [222] | Rudra A, Dutta S, Raju S V. The Paleogene vegetation and petroleum system in the tropics:a biomarker approach[J]. Marine and Petroleum Geology, 2017, 86: 38–51. DOI: 10.1016/j.marpetgeo.2017.05.008 |
| [223] | Jiang F J, Pang X Q, Bai J, et al. Comprehensive assessment of source rocks in the Bohai Sea area, eastern China[J]. AAPG Bulletin, 2016, 100(6): 969–1002. DOI: 10.1306/02101613092 |
| [224] | Al-Ameri T K, Al-Temimi A K, Zumberge J. Assessments of oil characterization, source affinities, and hydrocarbon dynamic of East Baghdad oil fields, Central Iraq[J]. Marine and Petroleum Geology, 2016, 77: 353–375. DOI: 10.1016/j.marpetgeo.2016.03.009 |
| [225] | Wang H Y, Liu W G, Lu H X. Appraisal of branched glycerol dialkyl glycerol tetraether-based indices for North China[J]. Organic Geochemistry, 2016, 98: 118–130. DOI: 10.1016/j.orggeochem.2016.05.013 |
| [226] | Schouten S, Hopmans E C, Schefube, et al. Distributional variations in marine crenarchaeotal membrane lipids:a new tool for reconstructing ancient sea water temperatures?[J]. Earth and Planetary Science Letters, 2002, 204(1/2): 265–274. |
| [227] | Mutterlose J, Malkoc M, Schouten S, et al. TEX86 and stable δ18O paleothermometry of early Cretaceous sediments:Implications for belemnite ecology and paleotemperature proxy application[J]. Earth and Planetary Science Letters, 2010, 298(3/4): 286–298. |
| [228] | Farquhar J, Bao H M, Thiemens M. Atmospheric influence of Earth's earliest sulfur cycle[J]. Science, 2000, 289(5480): 756–758. DOI: 10.1126/science.289.5480.756 |
| [229] | Frei R, Gaucher C, Poulton S W, et al. Fluctuations in Precambrian atmospheric oxygenation recorded by chromium isotopes[J]. Nature, 2009, 461(7261): 250–253. DOI: 10.1038/nature08266 |
| [230] | Rowley D B, Pierrehumbert R T, Currie B S. A new approach to stable isotope-based paleoaltimetry:implications for paleoaltimetry and paleohypsometry of the High Himalaya since the Late Miocene[J]. Earth and Planetary Science Letters, 2001, 188(1/2): 253–268. |
| [231] | Dale A, John C M, Mozley P S, et al. Time-capsule concretions:unlocking burial diagenetic processes in the Mancos Shale using carbonate clumped isotopes[J]. Earth and Planetary Science Letters, 2014, 394: 30–37. DOI: 10.1016/j.epsl.2014.03.004 |
| [232] | Shen B, Dong L, Xiao S H, et al. Molar tooth carbonates and benthic methane fluxes in Proterozoic oceans[J]. Nat Commun, 2016, 7: 10317. DOI: 10.1038/ncomms10317 |
| [233] | Shen Y N, Farquhar J, Zhang H, et al. Multiple S-isotopic evidence for episodic shoaling of anoxic water during Late Permian mass extinction[J]. Nature Communications, 2011, 2: 210. DOI: 10.1038/ncomms1217 |
| [234] | Teng F Z, Dauphas N, Watkins J M. Non-traditional stable isotopes:retrospective and prospective[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 1–26. DOI: 10.2138/rmg.2017.82.1 |
| [235] | Raiswell R, Canfield D E. Sources of iron for pyrite formation in marine sediments[J]. American Journal of Science, 1998, 298(3): 219–245. DOI: 10.2475/ajs.298.3.219 |
| [236] | Poulton S W, Canfield D E. Development of a sequential extraction procedure for iron:implications for iron partitioning in continentally derived particulates[J]. Chemical Geology, 2005, 214(3-4): 209–221. DOI: 10.1016/j.chemgeo.2004.09.003 |
| [237] | Li C, Love G D, Lyons T W, et al. A stratified redox model for the Ediacaran ocean[J]. Science, 2010, 328(5974): 80–83. DOI: 10.1126/science.1182369 |
| [238] | Li C, Jin C S, Planavsky N J, et al. Coupled oceanic oxygenation and metazoan diversification during the early-middle Cambrian?[J]. Geology, 2017, 45(8): 743–746. |
| [239] | Jin C S, Li C, Algeo T J, et al. A highly redox-heterogeneous ocean in South China during the early Cambrian (~529-514 Ma):implications for biota-environment co-evolution[J]. Earth and Planetary Science Letters, 2016, 441: 38–51. DOI: 10.1016/j.epsl.2016.02.019 |
| [240] | Wotte T, Shields-Zhou G A, Strauss H. Carbonate-associated sulfate:experimental comparisons of common extraction methods and recommendations toward a standard analytical protocol[J]. Chemical Geology, 2012, 326-327: 132–144. DOI: 10.1016/j.chemgeo.2012.07.020 |
| [241] | Theiling B P, Coleman M. Refining the extraction methodology of carbonate associated sulfate:evidence from synthetic and natural carbonate samples[J]. Chemical Geology, 2015, 411: 36–48. DOI: 10.1016/j.chemgeo.2015.06.018 |
| [242] | Swart P K. The geochemistry of carbonate diagenesis:the past, present and future[J]. Sedimentology, 2015, 62(5): 1233–1304. DOI: 10.1111/sed.12205 |
| [243] | Swart P K, Kennedy M J. Does the global stratigraphic reproducibility of δ13C in Neoproterozoic carbonates require a marine origin? A Pliocene-Pleistocene comparison[J]. Geology, 2012, 40(1): 87–90. DOI: 10.1130/G32538.1 |
| [244] | Prendergast A L, Versteegh E A A, Sch ne B R. New research on the development of high-resolution palaeoenvironmental proxies from geochemical properties of biogenic carbonates[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2017, 484: 1–6. DOI: 10.1016/j.palaeo.2017.05.032 |
| [245] | Dong S F, Chen D Z, Qing H R, et al. In situ stable isotopic constraints on dolomitizing fluids for the hydrothermally-originated saddle dolomites at Keping, Tarim Basin[J]. Chinese Science Bulletin, 2013, 58(23): 2877–2882. DOI: 10.1007/s11434-013-5801-7 |
| [246] | Zhou L L, Mckenna C A, Long D G F, et al. LA-ICP-MS elemental mapping of pyrite:an application to the Palaeoproterozoic atmosphere[J]. Precambrian Research, 2017, 297: 33–55. DOI: 10.1016/j.precamres.2017.05.008 |
| [247] | Shanahan T M, Overpeck J T, Hubeny J B, et al. Scanning micro-X-ray fluorescence elemental mapping:a new tool for the study of laminated sediment records[J]. Geochemistry, Geophysics, Geosystems, 2008, 9(2): Q02016. |
| [248] | Large R R, Halpin J A, Danyushevsky L V, et al. Trace element content of sedimentary pyrite as a new proxy for deep-time ocean-atmosphere evolution[J]. Earth and Planetary Science Letters, 2014, 389: 209–220. DOI: 10.1016/j.epsl.2013.12.020 |
| [249] | Gregory D D, Large R R, Halpin J A, et al. Trace element content of sedimentary pyrite in black shales[J]. Economic Geology, 2015, 110(6): 1389–1410. DOI: 10.2113/econgeo.110.6.1389 |
| [250] | Wacey D, Kilburn M R, Saunders M, et al. Uncovering framboidal pyrite biogenicity using nano-scale CNorg mapping[J]. Geology, 2014, 43(1): 27–30. |
| [251] | Peng X T, Ta K W, Chen S, et al. Coexistence of Fe(Ⅱ)-and Mn(Ⅱ)-oxidizing bacteria govern the formation of deep sea umber deposits[J]. Geochimica et Cosmochimica Acta, 2015, 169: 200–216. DOI: 10.1016/j.gca.2015.09.011 |
| [252] | Peng X T, Guo Z X, House C H, et al. SIMS and NanoSIMS analyses of well-preserved microfossils imply oxygen-producing photosynthesis in the Mesoproterozoic anoxic ocean[J]. Chemical Geology, 2016, 441: 24–34. DOI: 10.1016/j.chemgeo.2016.08.011 |
| [253] | Marshall C P, Emry J R, Marshall A O. Haematite pseudomicrofossils present in the 3.5-billion-year-old Apex Chert[J]. Nature Geoscience, 2011, 4(4): 240–243. DOI: 10.1038/ngeo1084 |
| [254] | Brasier M D, Antcliffe J, Saunders M, et al. Changing the picture of Earth's earliest fossils (3.5-1.9 Ga) with new approaches and new discoveries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(16): 4859–4864. DOI: 10.1073/pnas.1405338111 |
| [255] | Lin Z Y, Sun X M, Peckmann J, et al. How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite:a SIMS study from the South China Sea[J]. Chemical Geology, 2016, 440: 26–41. DOI: 10.1016/j.chemgeo.2016.07.007 |
| [256] | Chen J, Shen S Z, Li X H, et al. High-resolution SIMS oxygen isotope analysis on conodont apatite from South China and implications for the end-Permian mass extinction[J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2016, 448: 26–38. DOI: 10.1016/j.palaeo.2015.11.025 |
| [257] | Schmitz M D, Kuiper K F. High-precision geochronology[J]. Elements, 2013, 9(1): 25–30. DOI: 10.2113/gselements.9.1.25 |
| [258] | Rothman D H, Hayes J M, Summons R E. Dynamics of the Neoproterozoic carbon cycle[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(14): 8124–8129. DOI: 10.1073/pnas.0832439100 |
| [259] | Bjerrum C J, Canfield D E. Towards a quantitative understanding of the late Neoproterozoic carbon cycle[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(14): 5542–5547. DOI: 10.1073/pnas.1101755108 |
| [260] | Mcinerney F A, Wing S L. The paleocene-eocene thermal maximum:a perturbation of carbon cycle, climate, and biosphere with implications for the future[J]. Annual Review of Earth and Planetary Sciences, 2011, 39: 489–516. DOI: 10.1146/annurev-earth-040610-133431 |
| [261] | Meissner K J, Bralower T J, Alexander K, et al. The paleocene-eocene thermal maximum:how much carbon is enough?[J]. Paleoceanography, 2014, 29(10): 946–963. DOI: 10.1002/2014PA002650 |
| [262] | Frieling J, Svensen H H, Planke S, et al. Thermogenic methane release as a cause for the long duration of the PETM[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(43): 12059–12064. DOI: 10.1073/pnas.1603348113 |
| [263] | Hülse D, Arndt S, Wilson J D, et al. Understanding the causes and consequences of past marine carbon cycling variability through models[J]. Earth-Science Reviews, 2017, 171: 349–382. DOI: 10.1016/j.earscirev.2017.06.004 |
| [264] | Reinhard C T, Planavsky N J, Robbins L J, et al. Proterozoic ocean redox and biogeochemical stasis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(14): 5357–5362. DOI: 10.1073/pnas.1208622110 |
| [265] | Zhang S C, Wang X M, Wang H J, et al. Sufficient oxygen for animal respiration 1, 400 million years ago[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(7): 1731–1736. DOI: 10.1073/pnas.1523449113 |
| [266] | Algeo T J, Luo G M, Song H Y, et al. Reconstruction of secular variation in seawater sulfate concentrations[J]. Biogeosciences, 2015, 12(7): 2131–2151. DOI: 10.5194/bg-12-2131-2015 |
| [267] | Arning E T, Van Berk W, Schulz H M. Fate and behaviour of marine organic matter during burial of anoxic sediments:testing CH2O as generalized input parameter in reaction transport models[J]. Marine Chemistry, 2016, 178: 8–21. DOI: 10.1016/j.marchem.2015.12.002 |
| [268] | Meister P, Liu B, Ferdelman T G, et al. Control of sulphate and methane distributions in marine sediments by organic matter reactivity[J]. Geochimica et Cosmochimica Acta, 2013, 104: 183–193. DOI: 10.1016/j.gca.2012.11.011 |
| [269] | Dickens G R, Fewless T, Thomas E, et al. Excess barite accumulation during the Paleocene-Eocene thermal Maximum:massive input of dissolved barium from seafloor gas hydrate reservoirs[J]. Geological Society of America Special Papers, 2003, 369: 11–23. |
| [270] | Paraska D W, Hipsey M R, Salmon S U. Sediment diagenesis models:review of approaches, challenges and opportunities[J]. Environmental Modelling & Software, 2014, 61: 297–325. |
| [271] | Dickens G R. Sulfate profiles and barium fronts in sediment on the Blake Ridge:present and past methane fluxes through a large gas hydrate reservoir[J]. Geochimica et Cosmochimica Acta, 2001, 65(4): 529–543. DOI: 10.1016/S0016-7037(00)00556-1 |
| [272] | Malinverno A, Pohlman J W. Modeling sulfate reduction in methane hydrate-bearing continental margin sediments:does a sulfate-methane transition require anaerobic oxidation of methane?[J]. Geochemistry, Geophysics, Geosystems, 2011, 12(7): Q07006. |
| [273] | Veizer J, Ala D, Azmy K, et al. 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater[J]. Chemical Geology, 1999, 161(1/2/3): 59–88. |
| [274] | Shields G, Veizer J. Precambrian marine carbonate isotope database:version 1.1[J]. Geochemistry, Geophysics, Geosystems, 2002, 3(6): 1 of 12–12 of 12. DOI: 10.1029/2001GC000266 |
| [275] | Jaffrés J B D, Shields G A, Wallmann K. The oxygen isotope evolution of seawater:a critical review of a long-standing controversy and an improved geological water cycle model for the past 3.4 billion years[J]. Earth-Science Reviews, 2007, 83(1/2): 83–122. |
| [276] | Liu X M, Kah L C, Knoll A H, et al. Tracing Earth's O2 evolution using Zn/Fe ratios in marine carbonates[J]. Geochemical Perspectives Letters, 2016, 2(1): 24–34. DOI: 10.7185/geochemlet.1603 |
| [277] | Scott C, Lyons T W, Bekker A, et al. Tracing the stepwise oxygenation of the Proterozoic ocean[J]. Nature, 2008, 452(7186): 456–459. DOI: 10.1038/nature06811 |
| [278] | Sahoo S K, Planavsky N J, Kendall B, et al. Ocean oxygenation in the wake of the Marinoan glaciation[J]. Nature, 2012, 489(7417): 546–549. DOI: 10.1038/nature11445 |
| [279] | Partin C, Bekker A, Planavsky N J, et al. Large-scale fluctuations in Precambrian atmospheric and oceanic oxygen levels from the record of U in shales[J]. Earth and Planetary Science Letters, 2013, 369-370: 284–293. DOI: 10.1016/j.epsl.2013.03.031 |
| [280] | Stüeken E E, Buick R, Bekker A, et al. The evolution of the global selenium cycle:Secular trends in Se isotopes and abundances[J]. Geochimica et Cosmochimica Acta, 2015, 162: 109–125. DOI: 10.1016/j.gca.2015.04.033 |
| [281] | Konhauser K O, Pecoits E, Lalonde S V, et al. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event[J]. Nature, 2009, 458(7239): 750–753. DOI: 10.1038/nature07858 |
| [282] | Planavsky N J, Rouxel O J, Bekker A, et al. The evolution of the marine phosphate reservoir[J]. Nature, 2010, 467(7319): 1088–1090. DOI: 10.1038/nature09485 |
| [283] | Large R R, Halpin J A, Lounejeva E, et al. Cycles of nutrient trace elements in the Phanerozoic ocean[J]. Gondwana Research, 2015, 28(4): 1282–1293. DOI: 10.1016/j.gr.2015.06.004 |
| [284] | Large R R, Gregory D D, Steadman J A, et al. Gold in the oceans through time[J]. Earth and Planetary Science Letters, 2015, 428: 139–150. DOI: 10.1016/j.epsl.2015.07.026 |
| [285] | Algeo T J, Meyers P A, Robinson R S, et al. Icehouse-greenhouse variations in marine denitrification[J]. Biogeosciences, 2014, 11(4): 1273–1295. DOI: 10.5194/bg-11-1273-2014 |
| [286] | Johnson C M, Beard B L, Roden E E. The Iron isotope fingerprints of redox and biogeochemical cycling in modern and ancient earth[J]. Annual Review of Earth and Planetary Sciences, 2008, 36: 457–493. DOI: 10.1146/annurev.earth.36.031207.124139 |
| [287] | Farquhar J, Peters M, Johnston D T, et al. Isotopic evidence for Mesoarchaean anoxia and changing atmospheric sulphur chemistry[J]. Nature, 2007, 449(7163): 706–709. DOI: 10.1038/nature06202 |
| [288] | Vollstaedt H, Eisenhauer A, Wallmann K, et al. The Phanerozoic δ88/86Sr record of seawater:new constraints on past changes in oceanic carbonate fluxes[J]. Geochimica et Cosmochimica Acta, 2014, 128: 249–265. DOI: 10.1016/j.gca.2013.10.006 |
| [289] | Farkaš J, B hm F, Wallmann K, et al. Calcium isotope record of Phanerozoic oceans:Implications for chemical evolution of seawater and its causative mechanisms[J]. Geochimica et Cosmochimica Acta, 2007, 71(21): 5117–5134. DOI: 10.1016/j.gca.2007.09.004 |
| [290] | Lowenstein T K, Timofeeff M N, Brennan S T, et al. Oscillations in Phanerozoic seawater chemistry:evidence from fluid inclusions[J]. Science, 2001, 294(5544): 1086–1088. DOI: 10.1126/science.1064280 |
| [291] | Peucker-Ehrenbrink B, Ravizza G. The marine osmium isotope record[J]. Terra Nova, 2000, 12(5): 205–219. DOI: 10.1046/j.1365-3121.2000.00295.x |
| [292] | Berner R A. GEOCARBSULF:a combined model for Phanerozoic atmospheric O2 and CO2[J]. Geochimica et Cosmochimica Acta, 2006, 70(23): 5653–5664. DOI: 10.1016/j.gca.2005.11.032 |
| [293] | Lyons T W, Reinhard C T, Planavsky N J. The rise of oxygen in Earth's early ocean and atmosphere[J]. Nature, 2014, 506(7488): 307–315. DOI: 10.1038/nature13068 |
| [294] | Planavsky N J, Mcgoldrick P, Scott C T, et al. Widespread iron-rich conditions in the mid-Proterozoic ocean[J]. Nature, 2011, 477(7365): 448–451. DOI: 10.1038/nature10327 |
| [295] | Guilbaud R, Poulton S W, Butterfield N J, et al. A global transition to ferruginous conditions in the early Neoproterozoic oceans[J]. Nature Geoscience, 2015, 8(6): 466–470. DOI: 10.1038/ngeo2434 |
| [296] | Tostevin R, Wood R A, Shields G A, et al. Low-oxygen waters limited habitable space for early animals[J]. Nature Communications, 2016, 7: 12818. DOI: 10.1038/ncomms12818 |
| [297] | Jiang G Q, Kaufman A J, Christie-Blick N, et al. Carbon isotope variability across the Ediacaran Yangtze platform in South China:implications for a large surface-to-deep ocean δ13C gradient[J]. Earth and Planetary Science Letters, 2007, 261(1/2): 303–320. |
| [298] | Jiang G Q, Wang X Q, Shi X Y, et al. The origin of decoupled carbonate and organic carbon isotope signatures in the early Cambrian (ca. 542-520 Ma) Yangtze platform[J]. Earth and Planetary Science Letters, 2012, 317-318: 96–110. DOI: 10.1016/j.epsl.2011.11.018 |
| [299] | Li C, Hardisty D S, Luo G, et al. Uncovering the spatial heterogeneity of Ediacaran carbon cycling[J]. Geobiology, 2017, 15(2): 211–224. DOI: 10.1111/gbi.2017.15.issue-2 |
| [300] | Feng L J, Li C, Huang J, et al. A sulfate control on marine mid-depth euxinia on the early Cambrian (ca. 529-521 Ma) Yangtze platform, South China[J]. Precambrian Research, 2014, 246: 123–133. DOI: 10.1016/j.precamres.2014.03.002 |
 2017, Vol. 35
2017, Vol. 35