2. Beijing NMR Center, Peking University, Beijing 100871, China;
3. National Laboratories of Beijing National Laboratory for Molecular Science, Beijing 100871, China
2. 北京核磁共振中心, 北京 100871;
3. 北京分子科学国家实验室, 北京 100871
Membrane proteins do not stay isolated; they interact with other biomolecules, such as other membrane proteins, soluble proteins, lipids and small molecules, to carry out their biological functions[1-3]. Understanding the functions of membrane proteins requires a detailed characterization of their dynamic structures.
Among numerous biophysical tools, solid-state nuclear magnetic resonance (ssNMR) is an excellent choice for the study of membrane proteins in membranous environments[4-8]. While the traditional 1H, 15N and 13C ssNMR provide plenty of information about membrane protein structure, 19F ssNMR is an attractive technique in probing interactions within the membranes because of the excellent NMR properties of the 19F nucleus. The 19F nucleus with spin of 1/2 has a high gyromagnetic ratio. These two properties lead to a simple spectral line shape and high NMR sensitivity, which allows the detection of 19F compounds with only 0.5 mg materials. The 19F nucleus also possesses a wide chemical shift range of δ~400[9], which means that subtle changes in the local chemical environment might lead to chemical shift variations that are detectable with 19F ssNMR[10, 11]. Importantly, the total absence of fluorine in nature facilitates the easy identification of target 19F compounds in complex cellular environments, making 19F ssNMR suitable for in vivo studies.
Recently, 19F ssNMR has been used to elucidate protein assembles including parainfluenza virus fusion protein[12, 13], alamethicin dimers[14] and amyloid-like beta-sheet peptide[15], and to characterize orientation of peptides such as cyclotide kalata B1[16], antimicrobial peptide PGLa[17] and SB056[18]. 19F ssNMR has also been used for in vivo ssNMR. In this review, we summarize the recent progress using 19F ssNMR to probe interactions among proteins and other biomolecules in bio-membranes. We introduce labeling strategies, 19F ssNMR approaches and the impressive studies that have used 19F ssNMR to characterize protein interactions. We also briefly discuss in vivo applications of 19F ssNMR. Other recent reviews[19-25] have summarized the successful use of solution 19F NMR to characterize the structure and dynamics of soluble proteins, both in vitro and in vivo.
1 Preparation of 19 F-labeled proteinsThe 19F nucleus can be introduced into proteins using fluorinated amino acids or covalently attached to proteins by chemical modification of the thiol group of cysteine residues. The 19F-probes used for protein studies always contain either a mono F- or a CF3-reporter. Single fluorine is the simplest way of detecting change in the chemical environment, and the 19F-19F dipolar coupling within the CF3-triad could provide information about the orientation and dynamics of the target protein. Three strategies have typically been used to prepare fluorinated proteins: chemical synthesis, biosynthesis and chemical modifications, as shown in Fig. 1.
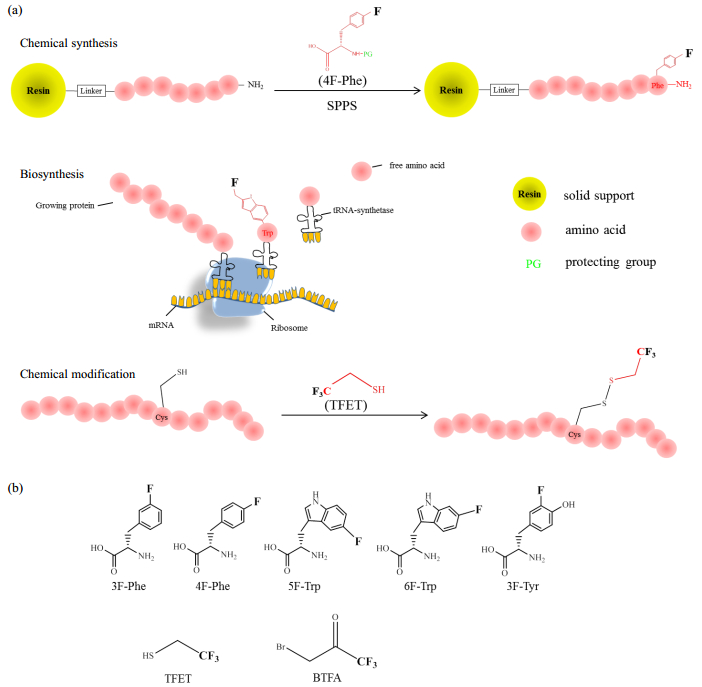
|
Fig. 1 (a) Schematic illustration of three labelling strategies for protein fluorination; (b) Commercially available F-probes: 3-fluoro-L-phenylalanine (3F-Phe), 4-fluoro-L-phenylalanine (4F-Phe), 5-fluoro-L-tryptophan (5F-Trp), 6-fluoro-L-tryptophan (6F-Trp), 3-fluoro-L-tyrosine (3F-Tyr), 2, 2, 2-trifluoroehanethiol (TFET), 3-bromo-1, 1, 1-trifluoroacetone (BTFA) |
Based on the solid-phase peptide synthesis (SPPS) method pioneered by Merrifield[26], peptide synthesis can be achieved by chemically coupling amino acids sequentially onto the solid supporter (resin). The SPPS process can now be performed using an automated synthesizer machine. With the automated SPPS method, fluorinated unnatural amino acids can be incorporated in opted positions. Commercially available fluorinated amino acids are mostly aromatic amino acids, such as 3F-Phe[27, 28], 4F-Phe[29, 30], 5F-Trp[31-33], 6F-Trp[34, 35] and 3F-Tyr[36-40]. Further separation and identification are necessary to characterize the racemization. It is worth noting that the SPPS method is practical for peptides that consist of less than 40 amino acid residues. For longer peptides, the coupling efficiency is dramatically reduced.
1.2 BiosynthesisBiosynthesis is potential to produce large proteins, and E.coli strains are often used as expression hosts. The DNA sequence for the protein or peptide of interest is usually carried by a plasmid and transformed into the hosts. The fluorine labels can be introduced into target proteins by feeding 19F-labeled unnatural amino acids, such as 5F-Trp, into the growth media[31-33]. The 19F-labeled unnatural amino acids are then absorbed by the host cells and incorporated into the expressed proteins at the positions of the native amino acids of the same type. This biosynthetic approach is convenient for generating residue-type specific 19F-labeling but faces challenges. The method can produce low yields due to the toxicity of fluorine and the inefficient uptake by the corresponding tRNA-synthetase, which results from the high competition among native amino acids from cellular environments.
Another biotechnical advance that is suitable for 19F-labeling is the use of cell-free systems to enable protein expression in cell extract systems[41, 42]. The fluorinated amino acids can be easily added into the open system and captured by the synthetase[43, 44].
1.3 Chemical modificationFluorination at aliphatic sites can be achieved in a protein by covalent chemical modification on the thiol group of cysteine residues. The fluorinated tags usually contain a trifluoromethyl-group and attach to cysteine by a disulfide or a S-C bond. For example, 2, 2, 2-trifluoroethanethiol (TFET)[11, 45] and 3-bromo-1, 1, 1-trifluoroacetone (BTFA) [46, 47] are two of the most commonly used 19F NMR probes due to their relatively small size. The chemical modification method results in straightforward labeling and might be applicable for in vivo studies.
2 19F ssNMR technique for membrane proteins studyBio-membranes provide a hydrophobic environment for proteins to fold into a specific structure. Membranes also offer an enclosed place for various biological interactions, which involve different types of proteins, i.e., A. antimicrobial peptides (AMPs), B. cell-penetrating peptides (CPPs), C. membrane channel protein, D. membrane fusion peptides (FP), E. membrane receptor protein, as shown in Fig. 2. Membrane-active proteins interact with other proteins in membrane environments, and ssNMR is an outstanding tool to study membrane-active proteins. The technique can determine the structure or topology of small peptides and can also address the local structure of large proteins. For example, ssNMR can determine the distances between selectively labeled sites on a protein, which facilitates the understanding of mechanisms for protein-protein or protein-ligand interactions. Also, the orientation and topology of membrane proteins and their interactions with lipids can be studied with ssNMR. A number of 19F ssNMR techniques have emerged to study protein structure, orientation and interactions.
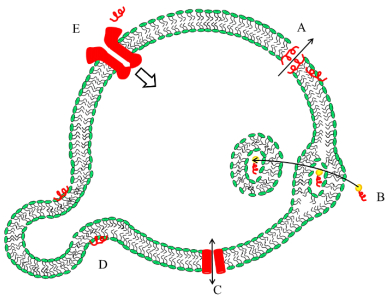
|
Fig. 2 Schematic illustration of the interactions within membranes. A: Antimicrobial peptides permeate the membranes by pore formation; B: The cell-penetrating peptides go through the cell membrane by direct translocation or endocytosis. They always participate in cargo delivery; C: Membrane ion channel protein; D: Fusion peptides facilitate membrane fusion; E: Membrane receptor protein can be activated by specific binding with molecules such as small peptides, which lead to signal transduction |
The orientation of membrane proteins in lipid bilayers is critical to understanding the topology, structure and function of membrane proteins. The orientation and dynamic behavior of membrane proteins in bio-membranes can be described by a set of constraints (Fig. 3). Using the α-helix as an example, those constraints include the tilt angle (τ) with respect to the membrane normal (N), the azimuthal rotational angle (ρ) that describes the tilt direction of the peptides and the order parameter (Smol) that represents the wobbling motion.
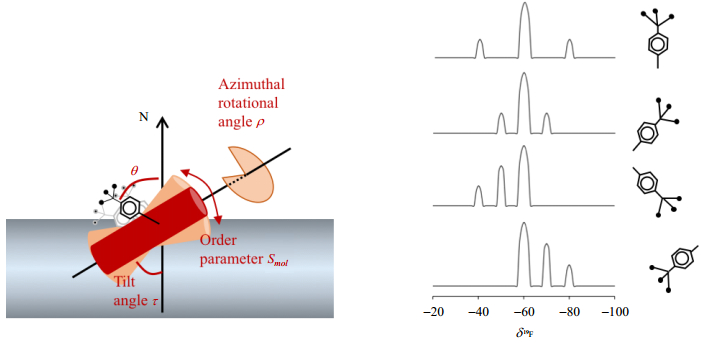
|
Fig. 3 Characterization of peptide orientation (τ, ρ and Smol) by CF3-probes |
The orientation information is commonly derived from static NMR experiments using uniaxially aligned samples, rather than the dipolar recoupling techniques under magic angle spinning (MAS) ssNMR. The CF3-group is an effective probe in terms of minimum material consume, maximum sensitivity and experimental ease. To obtain information on membrane alignment and motional dynamics, the CF3-probe needs to be rigidly attached to the peptides in a well-defined geometry, e.g., CF3-phenylglycine (CF3-Phg). A single pulse 19F NMR experiment allows the simultaneous detection of both the anisotropic chemical shifts and the homonuclear dipolar coupling within the CF3-group. The fast rotation of the CF3-group around the C-CF3 axis makes the three spins chemically and magnetically equivalent. The homonuclear dipolar interaction within the CF3-triad results in a triplet in the static 1H-decoupled 19F NMR spectrum. The splitting of the triplet depends on the angle θ between the C-CF3 axis and the external magnetic field B0, where θ is given by
| $ \mathit{\Delta }_{{\rm{FF}}}^0 = \mathit{\Delta }_{{\rm{FF}}}^{\max }(3{\cos ^2}\theta - 1)/2 $ | (1) |
When the CF3-axis aligns parallel to the membrane normal N, it theoretically produces the maximum intra-CF3 dipolar coupling, given by
| $ \mathit{\Delta }_{{\rm{FF}}}^{\max } = \frac{1}{2}\frac{{3{\gamma ^2}\hbar }}{{r_{{\rm{FF}}}^3}} = 8.{\rm{7 kHz}} $ | (2) |
The Δ and the r represent the dipolar couplings and the distance between the two 19F nuclei. Any additional motion to the CF3-rotation, such as axial rotation in the crystalline lipid membrane around the membrane normal, molecular wobble or diffusion, further scales the dipolar splitting by an order parameter, given by
| $ \mathit{\Delta }_{{\rm{FF}}}^{} = {S_{mol}}\mathit{\Delta }_{{\rm{FF}}}^0 $ | (3) |
and can be used to describe the molecular motional dynamics.
Obtaining the orientational constraints τ, ρ and
The distance between 19F nuclei can be measured based on 19F-19F dipolar interactions. The introduction of 19F-labels prolongs the detection distance to the maximum extent. The longest distance up to 17.5 Å (1 Å=0.1 nm), corresponding to a weak dipolar coupling of 30 Hz, can be obtained between 19F-19F, just less than 1H-1H. Approaches using ssNMR for distance measurements have recently been developed, and the basic concept underlying these ssNMR methods is the detection of distance-dependent mononuclear and heteronuclear dipolar couplings (~1/r3), spin diffusion (~1/r6) or the paramagnetic relaxation effect (PRE; ~1/r6). Typical NMR approaches for measuring distance are shown in Fig. 4.
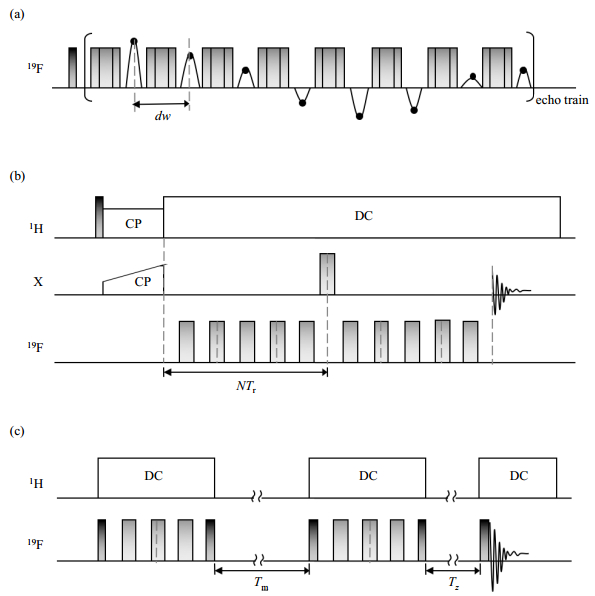
|
Fig. 4 Pulse sequences for measuring distance with ssNMR. (a) Car-Purcell-Meiboom-Gill (CPMG); (b) Rotational-echo double-resonance (REDOR); (c) Centerband-only detection of exchange (CODEX) |
The CPMG sequence is of great interest, as it is the unique approach for studies of protein interactions in fluid membranes under physiological conditions. The CPMG sequence utilizes a train of echo pulses after the first π/2 pulse, suppressing all interactions except homonuclear dipolar coupling[48-52]. Between every two successive π pulses, the echoes are recorded with a new dwell time (dw) as a function of the acquisition time. Fourier transformation gives the dipolar spectrum with pure homonuclear dipolar coupling.
2.2.2 Rotational-echo double-resonance (REDOR) experimentAs a representative method for detecting heteronuclear dipolar coupling, REDOR is a versatile sequence that can be adapted to measure all types of spin pairs. It is routinely used to measure 15N-13C spin pairs[53] and more recently 19F-involved spin pairs such as 31P-19F[54], 2H-19F[55], 13C-19F[56] and 15N-19F[57]. A REDOR experiment utilizes a train of rotor-synchronized 180° pulses on the dephasing channel (for an isolated X-19F spin pair, X to be obtained and 19F to be dephased), which can prevent the averaging of dipolar coupling by MAS and result in dephasing of the X magnetization transversed from the 1H by cross polarization (CP). The obtained spectrum is termed S and is followed by a reference spectrum S0 without the dephasing pulse trains. The plot of S/S0 as a function of the dephasing time can be fitted to obtain the inter-spin distances.
2.2.3 Centerband-only detection of exchange (CODEX) experimentCODEX experiment is based on the concept of 1H-driven spin diffusion under MAS strategy. It provides an attractive distance measurement approach because it measures the precise distance between selectively labeled sites and characterizes the oligomeric state[58]. The CODEX sequence contains a pair of rotor-synchronized π-pulse trains spaced by a mixing time (Tm), which is an integer number of the rotor period. The π-pulse trains recouple and enhance the chemical shift anisotropy (CSA) interactions. After the second 90˚ pulse, the magnetization flips back to the z-direction. During the Tm, the 19F-spins dephase, and this process is driven only by the 1H spin diffusion between the chemically equivalent but orientationally inequivalent spins. After the second π-pulse train but before acquisition, a short Tz is added. For each desired Tm, spectrum S and reference spectrum S0 are acquired with and interchanged Tm and Tz, and spectrum S will show reduced intensities. The CODEX curves are plotted as the value of S/S0 against the Tm. If an oligomer bundle exists, the curve decays to an equilibrium value of 1/m, where m indicates the oligomer number, and the rate of decay can reveal the intermolecular distances. CODEX experiments have been carried out at extremely low temperatures (e.g., 240 K), at which all the molecular motions are frozen and only distance-dependent spin diffusion occurs. The unwanted aggregation or denaturation of proteins at such low temperatures needs to be avoided.
2.2.4 Paramagnetic relaxation enhancement (PRE) experimentsNMR experiments using PRE are a feasible alternative to obtain distances (12~24 Å) that exceed the threshold of the other NMR approaches[59]. The T2 relaxation rate of 19F has been recorded to extract the average distances between 19F nuclei and the paramagnetic center [e.g., a S-(1-oxyl-2, 2, 5, 5-tetramethyl-2, 5-dihydro-1H-pyrrol-3-yl)methyl methanesulfonothioate (MTSL) spin label].
3 Structural insights into membrane proteins using 19F ssNMR 3.1 Probing peptide-membrane interactions by orientational determination: antimicrobial peptide PGLaAntimicrobial peptides (AMP) are one of the most concerning membrane-active polypeptides and can be found in almost all kinds of living organisms. AMPs kill microorganisms by physical disruption of the membrane integrity. These peptides could be a new type of antimicrobial drug with a low risk of drug resistance. The peptide PGLa belongs to the family of AMPs and is a distinct member of the magainin family, which has broad-spectrum antimicrobial activity. The structure and biological function of PGLa have been extensively studied. The antimicrobial mechanism has been suggested to permeabilize the microbial membranes by pore-formation[60-65], as shown in Fig. 2, and recent advances in understanding the action mechanism have been included in the review by Bechinger[66].
An accurate orientational and dynamic analysis of PGLa has been carried out with only four single CF3-Phg labeled PGLa mutants[67]. The orientational constraints can be extracted from the spectra using best fits, and the experimental data can be compared to simulated values, which are obtained by rotating the peptide molecule through all possible orientations [Fig. 5(a)~(c)]. The obtained orientational constraints (τ = 89˚, ρ= 106˚ and Smol = 0.6) agreed with previous 15N NMR data on PGLa, with the expected surface-bound "S-state" alignment at a peptide to lipid ratio of 1:200[68]. By increasing the peptide concentration, the peptide can tilt with the C-terminus into the membrane as a "T-state" by self-assembly as dimers, or it can insert into the membrane in a nearly standing "I-state"[67, 68]. The orientation of the peptide PGLa is also temperature dependent[69]; it was found to adopt an S-state above 45 ℃, a T-state with reduced temperature and even an I-state below 25 ℃ [Fig. 5(d)~(g)].
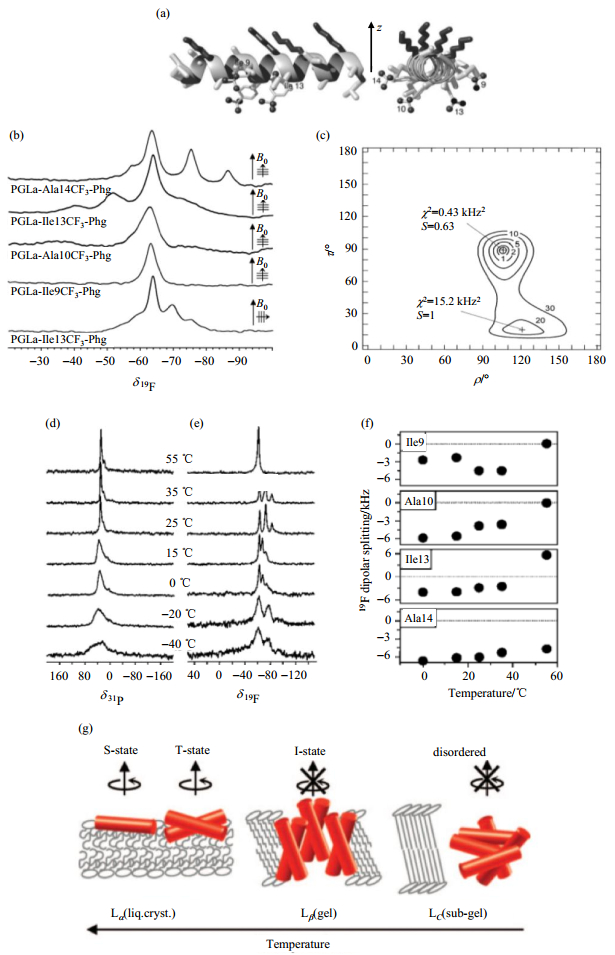
|
Fig. 5 Representative scheme and spectra for PGLa labeled with CF3-Phg at positions Ile9, Ala10, Ile13 and Ala14 around the helix. Macroscopically oriented samples are prepared with the peptide embedded in DMPC at a peptide to lipid ratio of 1:200. (b) Single 1-pulse 19F-spectra of the four PGLa mutants provide information about both the anisotropic chemical shifts and homonuclear dipolar couplings within the CF3-group. (c) Comparing the experimental data and simulated values, obtained by rotating the peptides through all possible orientations, showed the best fits of τ, ρ and Smol with a minimum χ2. (d & e) The temperature dependence of 31P-spectra (d) and 19F-spectra (e) of PGLa labeled with CF3-Phg at position Ile9. (f) 19F-dipolar coupling of all four PGLa mutants and a peptide to lipid ratio of 1:50 was utilized. (g) Four different states of PGLa were found, depending on the temperature. (a~g are adapted from Refs. [67] and [69]) |
Protein-protein and protein-peptide interactions include mono- and hetero-oligomerization through hydrophobic effects or electrostatic interactions that often happen within the lipid membrane or on the membrane surface. The determination of the local structure parameters in complex biological assemblies can be achieved by distance measurements. The 19F-labels incorporated into postulated oligomer interfaces allow for the exploration of possible contacts by detecting the spin diffusion of the 19F-isotopes or dipolar couplings between fluorine and other isotopes.
The M2 proton channel is an essential component in the envelope of the influenza A virus, which forms highly selective, proton-conducting channels across the membrane. It forms homotetramers in vivo[70] and in micelles[71]. Direct evidence about the oligomer state was first provided by 19F CODEX experiments. The M2 transmembrane segment was labeled with 4F-Phe in position Phe30 and then reconstituted into DMPC lipid bilayers at a peptide to lipid ratio of 1:15. The CODEX curve was obtained by fitting the experimental points to exponential curves. The curves decay to an equilibrium value of 1/4, which corresponds to an oligomer number of 4. The distances between distinct 19F-residues can be then obtained by analyzing the decay rate. The CODEX data revealed that the transmembrane segment of the M2 protein of the influenza A virus forms tetramers in DMPC bilayers with a nearest interhelical distance of 7.9~9.5 Å[58] (Fig. 6). Such an interhelical distance of 7.9~9.5 Å between the Phe30 residues suggests that the side chain conformation of Phe30 restricts the tilt angle of the helix to be over 20˚ and also defines the diameter of the tetramer channel.
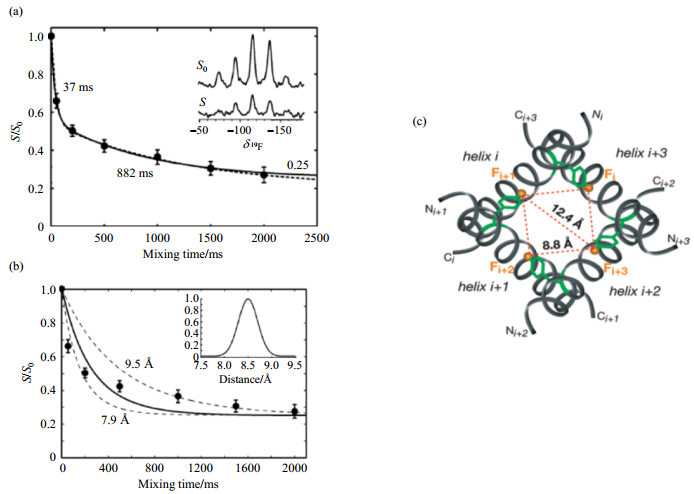
|
Fig. 6 CODEX experiments on the M2 protein in DMPC membranes[58]. (a) The CODEX curve drawn by fitting the experimental data with a biexponential decays to an equilibrium value of 1/4, indicating a tetramer bundle of the M2 proton channel protein. (b) The nearest 19F-19F distance of 7.9~9.5 Å can be given by a Gaussian distribution curve (inset) with a center of 8.5 Å (solid line), which shows a balanced fit (dashed lines) to both the short and long mixing time points. (c) The top view of the simulated tetramer model of the M2 TM segment labeled with a single 5F-Trp, showing a possible 19F-19F distance of 8.8 Å and 12.4 Å between adjacent helices, in agreement with the CODEX experimental results |
Proteins might behave differently in native cell environments than in solution or artificial phospholipids[72, 73]. As reported by Ulrich using the antimicrobial peptides PGLa and Gramicidin S, no distinct membrane tilted or inserted state was detected in native cells even though these states were found in artificial model membranes[74]. Findings like this one indicate that studying proteins inside living cells is becoming more attractive for biophysicists. One prerequisite for cellular NMR experiments is uniform-labeling with 13C and 15N or selective labeling with 19F, which has been successfully explored in E. coli overexpression systems and also eukaryotic systems[75-78]. 13C or 15N enrichment joined with solution NMR or high-resolution MAS ssNMR has been used for obtaining protein structures in the cellular context[79-82].
Routine 13C or 15N enrichment is often insufficient for the detection of globular proteins, probably due to the high viscosity and weak nonspecific intracellular interactions of the proteins[83]. In addition, the detection could be hampered by the intrinsic low sensitivity and high background of the 13C and 15N isotopes. These problems make the 19F to be a better choice in studying the globular and intrinsically disordered proteins, taking advantages of its total absence from biology and high sensitivity. The first application of 19F labeling for in-cell NMR experiments was in 1989. It used fluorinated phosphoglycerate kinase that was obtained by feeding 5F-Trp during enzyme synthesis in yeast. Two signals were detected and attributed to the two Trp residues in the protein[84]. 19F in-cell NMR methods have also been developed to monitor cellular biological processes. For example, Okamura and colleagues observed three states of the 19F-labeled peptide R8 in living cells and confirmed the spontaneous penetration of cell-penetrating peptides (CPPs) into cell membranes[85]. Tian and colleagues monitored the Tyr phosphorylation of Etk in lyophilized E.coli using the 19F ssNMR probe of 3, 5-difluorotyrosine incorporated at the position Tyr574 in the α-loop[86].
4 Limitations and future perspectivesThere are many fluorinated amino acids that have been developed for structural and functional studies of proteins[87-91]. Besides the NMR approaches mentioned above, 19F ssNMR also provides simple and straightforward way monitoring membrane protein interactions by detecting chemical shifts[92]. Despite the successes of applications of 19F in biological systems, it has an important potential limitation: the structural perturbations induced by the introduction of the unnatural 19F amino acid residues create a steric effect. The fluorination is always performed by the substitution of hydrogen, as the 19F nucleus is often considered to be isosteric with hydrogen because of their comparable van der Waal's radius. However, fluorine has the highest electronegativity of all the elements, at 3.98 on the Pauling scale[93]. On one hand, the fluorine substitution results in a C-F bond that is stronger than the C-H bond, which is stable throughout peptide synthesis or protein bio-expression. On the other hand, substitution of hydrogen for fluorine affects the electronic properties of the protein. The fluorine atom can donate electrons, while the hydrogen atom works as electron-acceptor. While many studies have shown that the introduction of a limited number of 19F-labels do not perturb the global structure, however, the unwanted steric and electronic effects cannot be ignored, especially in cases involving fluorine and hydroxyl interactions or the substituted sites locating in tightly packed protein cores. In such cases, even one single fluorine could cause significant changes to the protein structure[94]. On the other hand, novel fluorine labels need to be developed to meet a wide range of experimental requirements.
| [1] | ANDERSEN O S, KOEPPE R E. Ⅱ. Bilayer thickness and membrane protein function:An energetic perspective[J]. Annu Rev Biophys Biomol Struct, 2007, 36: 107-130. DOI: 10.1146/annurev.biophys.36.040306.132643. |
| [2] | PATCHING S G. Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery[J]. Biochim Biophys Acta, 2014, 1838(1Pt A): 43-55. |
| [3] | PHILLIPS R, URSELL T, WIGGINS P, et al. Emerging roles for lipids in shaping membrane-protein function[J]. Nature, 2009, 459(7245): 379-385. DOI: 10.1038/nature08147. |
| [4] | BAKER L A, BALDUS M. Characterization of membrane protein function by solid-state NMR spectroscopy[J]. Curr Opin Struc Biol, 2014, 27: 48-55. DOI: 10.1016/j.sbi.2014.03.009. |
| [5] | BAKER L A, FOLKERS G E, SINNIGE T, et al. Magic-angle-spinning solid-state nmr of membrane proteins[M]//SHUKLA A K. Membrane proteins-engineering, purification and crystallization. 2015, 557: 307-328. |
| [6] | HANSEN S K, BERTELSEN K, PAASKE B, et al. Solid-state NMR methods for oriented membrane proteins[J]. Prog Nucl Mag Res Sp, 2015(88/89): 48-85. |
| [7] | NAITO A, KAWAMURA I, JAVKHLANTUGS N. Recent solid-state NMR studies of membrane-bound peptides and proteins[J]. Annu Rep NMR Spectro, 2015, 86: 333-411. DOI: 10.1016/bs.arnmr.2015.06.001. |
| [8] | WARD M E, BROWN L S, LADIZHANSKY V. Advanced solid-state NMR techniques for characterization of membrane protein structure and dynamics:Application to anabaena sensory rhodopsin[J]. J Magn Reson, 2015, 253: 119-128. DOI: 10.1016/j.jmr.2014.11.017. |
| [9] | GERIG J T. Fluorine NMR[M/OL]. 2001. http://www.biophysics.org/img/jtg2001-2.pdf. |
| [10] | AASHISH M, TAE HUM KIM, MATTHIEU M, et al. Structural insights into the dynamic process of beta2-adrenergic receptor signaling[J]. Cell, 2015, 161(5): 1101-1111. DOI: 10.1016/j.cell.2015.04.043. |
| [11] | LIU J J, HORST R, KATRITCH V, et al. Biased signaling pathways in beta(2)-adrenergic receptor characterized by 19F NMR[J]. Science, 2012, 335(6072): 1106-1110. DOI: 10.1126/science.1215802. |
| [12] | LEE M, YAO H, KWON B, et al. Conformation and trimer association of the transmembrane domain of the parainfluenza virus fusion protein in lipid bilayers from solid-state nmr:insights into the sequence determinants of trimer structure and fusion activity[J]. J Mol Biol, 2018, 15: 695-709. |
| [13] | WILLIAMS J K, SHCHERBAKOV A A, WANG J, et al. Protonation equilibria and pore-opening structure of the dual-histidine influenza B virus M2 transmembrane proton channel from solid-state NMR[J]. J Biol Chem, 2017, 292(43): 17876-17884. DOI: 10.1074/jbc.M117.813998. |
| [14] | SALNIKOV E S, RAYA J, DE ZOTTI M, et al. Alamethicin supramolecular organization in lipid membranes from 19F solid-state NMR[J]. Biophys J, 2016, 111(11): 2450-2459. DOI: 10.1016/j.bpj.2016.09.048. |
| [15] | WADHWANI P, STRANDBERG E, HEIDENREICH N, et al. Self-assembly of flexible beta-strands into immobile amyloid-like beta-sheets in membranes as revealed by solid-state 19F NMR[J]. J Am Chem Soc, 2012, 134(15): 6512-6515. DOI: 10.1021/ja301328f. |
| [16] | GRAGE S L, SANI M A, CHENEVAL O, et al. Orientation and location of the cyclotide kalata b1 in lipid bilayers revealed by solid-state NMR[J]. Biophys J, 2017, 112(4): 630-642. DOI: 10.1016/j.bpj.2016.12.040. |
| [17] | MISIEWICZ J, AFONIN S, ULRICH A S. Control and role of pH in peptide-lipid interactions in oriented membrane samples[J]. Biochim Biophys Acta, 2015, 1848(3): 833-841. DOI: 10.1016/j.bbamem.2014.12.006. |
| [18] | MANZO G, SCORCIAPINO M A, WADHWANI P, et al. Enhanced amphiphilic profile of a short beta-stranded peptide improves its antimicrobial activity[J]. Plos One, 2015, 10(1): e0116379. DOI: 10.1371/journal.pone.0116379. |
| [19] | BATTISTE J, NEWMARK R A. Applications of 19F multidimensional NMR[J]. Prog Nucl Mag Res Sp, 2006, 48(1): 1-23. |
| [20] | KITEVSKI-LEBLANC J L, PROSSER R S. Current applications of 19F NMR to studies of protein structure and dynamics[J]. Prog Nucl Mag Res Sp, 2012, 62: 1-33. DOI: 10.1016/j.pnmrs.2011.06.003. |
| [21] | YU J X, HALLAC R R, CHIGURU S, et al. New frontiers and developing applications in 19F NMR[J]. Prog Nucl Mag Res Sp, 2013, 70: 25-49. DOI: 10.1016/j.pnmrs.2012.10.001. |
| [22] |
DAI C Y, LIU M L, LI C G. Salt content-dependent conformational changes of alpha-synuclein studied by 19F NMR[J].
Chinese J Magn Reson, 2015, 32(1): 33-40.
戴晨晔, 刘买利, 李从刚. 低盐和高盐环境下α-Synuclein构像的19F NMR[J]. 波谱学杂志, 2015, 32(1): 33-40. |
| [23] |
LI L S, LI Y, LAN Y J, et al. A brief review on 19F NMR[J].
Chinese J Magn Reson, 2007, 24(3): 353-364.
李临生, 李燕, 兰云军, 等. 19F NMR的特点[J]. 波谱学杂志, 2007, 24(3): 353-364. |
| [24] | LI C G, WANG G F, WANG Y Q, et al. Protein 19F NMR in Escherichia coli[J]. J Am Chem Soc, 2010, 132(1): 321-327. DOI: 10.1021/ja907966n. |
| [25] | YE Y S, LIU X L, ZHANG Z T, et al. 19F NMR spectroscopy as a probe of cytoplasmic viscosity and weak protein interactions in living cells[J]. Chemistry, 2013, 19(38): 12705-12710. DOI: 10.1002/chem.201301657. |
| [26] | MERRIFIELD R B. Solid phase peptide synthesis.1. Synthesis of a tetrapeptide[J]. J Am Chem Soc, 1963, 85(14): 2149-2154. DOI: 10.1021/ja00897a025. |
| [27] | LARDA S T, SIMONETTI K, AL-ABDUL-WAHID M S, et al. Dynamic equilibria between monomeric and oligomeric misfolded states of the mammalian prion protein measured by 19F NMR[J]. J Am Chem Soc, 2013, 135(28): 10533-10541. DOI: 10.1021/ja404584s. |
| [28] | LUCK L A, FALKE J J. 19F NMR-studies of the D-galactose chemosensory receptor.1. Sugar binding yields a global structural change[J]. Biochemistry, 1991, 30(17): 4248-4256. DOI: 10.1021/bi00231a021. |
| [29] | LI H, FRIEDEN C. NMR studies of 4-19F-phenylalanine-labeled intestinal fatty acid binding protein:Evidence for conformational heterogeneity in the native state[J]. Biochemistry, 2005, 44(7): 2369-2377. DOI: 10.1021/bi047600l. |
| [30] | LI H L, FRIEDEN C. Observation of sequential steps in the folding of intestinal fatty acid binding protein using a slow folding mutant and 19F NMR[J]. P Natl Acad Sci USA, 2007, 104(29): 11993-11998. DOI: 10.1073/pnas.0705253104. |
| [31] | CHADEGANI F, LOVELL S, MULLANGI V, et al. 19F nuclear magnetic resonance and crystallographic studies of 5-fluorotryptophan-labeled anthrax protective antigen and effects of the receptor on stability[J]. Biochemistry, 2014, 53(4): 690-701. DOI: 10.1021/bi401405s. |
| [32] | EVANICS F, BEZSONOVA I, MARSH J, et al. Tryptophan solvent exposure in folded and unfolded states of an SH3 domain by 19F and H-1 NMR[J]. Biochemistry, 2006, 45(47): 14120-14128. DOI: 10.1021/bi061389r. |
| [33] | ANDERLUH G, RAZPOTNIK A, PODLESEK Z, et al. Interaction of the eukaryotic pore-forming cytolysin equinatoxin Ⅱ with model membranes:19F NMR studies[J]. J Mol Biol, 2005, 347(1): 27-39. DOI: 10.1016/j.jmb.2004.12.058. |
| [34] | LI H, FRIEDEN C. Comparison of C40/82A and P27A C40/82A barstar mutants using 19F NMR[J]. Biochem, 2007, 46(14): 4337-4347. DOI: 10.1021/bi6026083. |
| [35] | BANN J G, PINKNER J, HULTGREN S J, et al. Real-time and equilibrium 19F NMR studies reveal the role of domain-domain interactions in the folding of the chaperone PapD[J]. P Natl Acad Sci USA, 2002, 99(2): 709-714. DOI: 10.1073/pnas.022649599. |
| [36] | LI C G, LUTZ E A, SLADE K M, et al. 19F NMR studies of alpha-synuclein conformation and fibrillation[J]. Biochemistry, 2009, 48(36): 8578-8584. DOI: 10.1021/bi900872p. |
| [37] | POMERANTZ W C, WANG N K, LIPINSKI A K, et al. Profiling the dynamic interfaces of fluorinated transcription complexes for ligand discovery and characterization[J]. ACS Chem Biol, 2012, 7(8): 1345-1350. DOI: 10.1021/cb3002733. |
| [38] | IRA J R, JOSHUA A B, PAULA M D. A residual structure in unfolded intestinal fatty acid binding protein consists of amino acids that are neighbors in the native state[J]. Biochem, 2006, 45(8): 2608-2617. DOI: 10.1021/bi052091o. |
| [39] | SCHLESINGER A P, WANG Y Q, TADEO X, et al. Macromolecular crowding fails to fold a globular protein in cells[J]. J Am Chem Soc, 2011, 133(21): 8082-8085. DOI: 10.1021/ja201206t. |
| [40] | ZIGONEANU I G, PIELAK G J. Interaction of alpha-synuclein and a cell penetrating fusion peptide with higher eukaryotic cell membranes assessed by 19F NMR[J]. Mol Pharm, 2012, 9(4): 1024-1029. DOI: 10.1021/mp200615m. |
| [41] | VILLARREAL F, TAN C. Cell-free systems in the new age of synthetic biology[J]. Front Chem Sci Eng, 2017, 11(1): 58-65. DOI: 10.1007/s11705-017-1610-x. |
| [42] | CARLSON E D, GAN R, HODGMAN C E, et al. Cell-free protein synthesis:Applications come of age[J]. Biotechnol Adv, 2012, 30(5): 1185-1194. DOI: 10.1016/j.biotechadv.2011.09.016. |
| [43] | HARADA R, FURUMOTO S, YOSHIKAWA T, et al. Synthesis and characterization of 18F-interleukin-8 using a cell-free translation system and 4-F-18-fluoro-l-proline[J]. J Nucl Med, 2016, 57(4): 634-639. DOI: 10.2967/jnumed.115.162602. |
| [44] | NEERATHILINGAM M, GREENE L, COLEBROOKE S, et al. Quantitation of protein expression in a cell-free system:efficient detection of yields and 19F NMR to identify folded protein[J]. J Biomol NMR, 2005, 31(1): 11-19. DOI: 10.1007/s10858-004-5357-6. |
| [45] | KLEIN-SEETHARAMAN J, GETMANOVA E V, LOEWEN M C, et al. NMR spectroscopy in studies of light-induced structural changes in mammalian rhodopsin:Applicability of solution 19F NMR[J]. P Natl Acad Sci USA, 1999, 96(24): 13744-13749. DOI: 10.1073/pnas.96.24.13744. |
| [46] | LUCHETTE P A, PROSSER R S, SANDERS C R. Oxygen as a paramagnetic probe of membrane protein structure by cysteine mutagenesis and 19F NMR spectroscopy[J]. J Am Chem Soc, 2002, 124(8): 1778-1781. DOI: 10.1021/ja016748e. |
| [47] | KIM TH, CHUNG K Y, MANGLIK A, et al. The role of ligands on the equilibria between functional states of a g protein-coupled receptor[J]. J Am Chem Soc, 2013, 135(25): 9465-9474. DOI: 10.1021/ja404305k. |
| [48] | MEIBOOM S, GILL D. Modified spin-echo method for measuring nuclear relaxation times[J]. Rev Sci Instr, 1958, 29(8): 688-691. DOI: 10.1063/1.1716296. |
| [49] | GRAGE S L, ULRICH A S. Structural parameters from 19F homonuclear dipolar couplings, obtained by multipulse solid-state NMR on static and oriented systems[J]. J Magn Reson, 1999, 138(1): 98-106. DOI: 10.1006/jmre.1999.1726. |
| [50] | SALGADO J, GRAGE S L, KONDEJEWSKI L H, et al. Membrane-bound structure and alignment of the antimicrobial beta-sheet peptide gramicidin S derived from angular and distance constraints by solid state 19F NMR[J]. J Biomol NMR, 2001, 21(3): 191-208. DOI: 10.1023/A:1012946026231. |
| [51] | GRAGE S L, SULEYMANOVA A V, AFONIN S, et al. Solid state NMR analysis of the dipolar couplings within and between distant CF3-groups in a membrane-bound peptide[J]. J Magn Reson, 2006, 183(1): 77-86. DOI: 10.1016/j.jmr.2006.07.012. |
| [52] | GRAGE S L, XU X J, SCHMITT M, et al. 19F-labeling of peptides revealing long-range nmr distances in fluid membranes[J]. J Phys Chem Lett, 2014, 5(24): 4256-4259. DOI: 10.1021/jz502195t. |
| [53] | NAITO A, NISHIMURA K, KIMURA S, et al. Determination of the three-dimensional structure of a new crystalline form of N-acetyl-Pro-Gly-Phe as revealed by 13C REDOR, X-ray diffraction, and molecular dynamics calculation[J]. J Phys Chem, 1996, 100(36): 14995-5004. DOI: 10.1021/jp960179t. |
| [54] | MERRITT M E, SIGURDSSON S T, DROBNY G P. Long-range distance measurements to the phosphodiester backbone of solid nucleic acids using 31P-19F REDOR NMR[J]. J Am Chem Soc, 1999, 121(25): 6070-6071. DOI: 10.1021/ja984173o. |
| [55] | GRAGE S L, WATTS J A, WATTS A. 2H{19F} REDOR for distance measurements in biological solids using a double resonance spectrometer[J]. J Magn Reson, 2004, 166(1): 1-10. |
| [56] | ANTONIOLI G, HODGKINSON P. Resolution of 13C-19F interactions in the 13C NMR of spinning solids and liquid crystals[J]. J Magn Reson, 2004, 168(1): 124-131. DOI: 10.1016/j.jmr.2004.02.004. |
| [57] | KIM S J, CEGELSKI L, PREOBRAZHENSKAYA M, et al. Structures of Staphylococcus aureus cell-wall complexes with vancomycin, eremomycin, and chloroeremomycin derivatives by 13C{19F} and 15N{19F} rotational-echo double resonance[J]. Biochemistry, 2006, 45(16): 5235-5250. DOI: 10.1021/bi052660s. |
| [58] | LUO W B, HONG M. Determination of the oligomeric number and intermolecular distances of membrane protein assemblies by anisotropic (1)H-driven spin diffusion NMR spectroscopy[J]. J Am Chem Soc, 2006, 128(22): 7242-7251. DOI: 10.1021/ja0603406. |
| [59] | MATEI E, GRONENBORN A M. 19F Paramagnetic relaxation enhancement:A valuable tool for distance measurements in proteins[J]. Angew Chem Int Ed, 2016, 55(1): 150-54. DOI: 10.1002/anie.201508464. |
| [60] | HUANG H W. Action of antimicrobial peptides:Two-state model[J]. Biochemistry, 2000, 39(29): 8347-8352. DOI: 10.1021/bi000946l. |
| [61] | SHAI Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides[J]. Biochim Biophys Acta, 1999, 1462(1/2): 55-70. |
| [62] | VAN'T HOF W, VEERMAN ECI, HELMERHORST E J, et al. Antimicrobial peptides:Properties and applicability[J]. Biol Chem, 2001, 382(4): 597-619. |
| [63] | EPAND R M, VOGEL H J. Diversity of antimicrobial peptides and their mechanisms of action[J]. Biochim Biophys Acta, 1999, 1462(1/2): 11-28. |
| [64] | BROGDEN K A. Antimicrobial peptides:Pore formers or metabolic inhibitors in bacteria?[J]. Nat Rev Microbiol, 2005, 3(3): 238-250. DOI: 10.1038/nrmicro1098. |
| [65] | OREN Z, SHAI Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides[J]. Biopolymers, 1998, 47(6): 451-463. DOI: 10.1002/(SICI)1097-0282(1998)47:6<>1.0.CO;2-W. |
| [66] | BECHINGER B, GORR S U. Antimicrobial peptides:mechanisms of action and resistance[J]. J Dent Res, 2017, 96(3): 254-260. DOI: 10.1177/0022034516679973. |
| [67] | GLASER R W, SACHSE C, DURR U H N, et al. Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F NMR dipolar couplings of 4-CF3-phenylglycine labels[J]. J Magn Reson, 2004, 168(1): 153-163. DOI: 10.1016/j.jmr.2004.02.008. |
| [68] | BECHINGER B, ZASLOFF M, OPELLA S J. Structure and dynamics of the antibiotic peptide PGLa in membranes by solution and solid-state nuclear magnetic resonance spectroscopy[J]. Biophy J, 1998, 74(2): 981-987. DOI: 10.1016/S0006-3495(98)74021-4. |
| [69] | AFONIN S, GRAGE SL, IERONIMO M, et al. Temperature-dependent transmembrane insertion of the amphiphilic peptide pgla in lipid bilayers observed by solid state 19F NMR spectroscopy[J]. J Am Chem Soc, 2008, 130(49): 16512-16514. DOI: 10.1021/ja803156d. |
| [70] | TANG Y J, ZAITSEVA F, LAMB R A, et al. The gate of the influenza virus M-2 proton channel is formed by a single tryptophan residue[J]. J Biol Chem, 2002, 277(42): 39880-39886. DOI: 10.1074/jbc.M206582200. |
| [71] | SALOM D, HILL B R, LEAR J D, et al. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus[J]. Biochemistry, 2000, 39(46): 14160-14170. DOI: 10.1021/bi001799u. |
| [72] | SPITZER J, POOLMAN B. The role of biomacromolecular crowding, ionic strength, and physicochemical gradients in the complexities of life's emergence[J]. Microbiol Mol Biol R, 2009, 73(2): 371-388. DOI: 10.1128/MMBR.00010-09. |
| [73] | MIKLOS A C, LI C, SHARAF N G, et al. Volume exclusion and soft interaction effects on protein stability under crowded conditions[J]. Biochemistry, 2010, 49(33): 6984-6991. DOI: 10.1021/bi100727y. |
| [74] | KOCH K, AFONIN S, IERONIMO M, et al. Solid-state 19F NMR of peptides in native membranes[J]. Top Curr Chem, 2012, 306: 89-118. |
| [75] | BERTRAND K, REVERDATTO S, BURZ D S, et al. Structure of proteins in eukaryotic compartments[J]. J Am Chem Soc, 2012, 134(30): 12798-12806. DOI: 10.1021/ja304809s. |
| [76] | HAMATSU J, O'DONOVAN D, TANAKA T, et al. High-resolution heteronuclear multidimensional NMR of proteins in living insect cells using a baculovirus protein expression system[J]. J Am Chem Soc, 2013, 135(5): 1688-1691. DOI: 10.1021/ja310928u. |
| [77] | BANCI L, BARBIERI L, BERTINI I, et al. Atomic-resolution monitoring of protein maturation in live human cells by NMR[J]. Nat Chem Biol, 2013, 9(5): 297-299. DOI: 10.1038/nchembio.1202. |
| [78] | BANCI L, BARBIERI L, LUCHINAT E, et al. Visualization of redox-controlled protein fold in living cells[J]. Chem Biol, 2013, 20(6): 747-752. DOI: 10.1016/j.chembiol.2013.05.007. |
| [79] | RAHMAN S, BYUN Y, HASSAN MI, et al. Towards understanding cellular structure biology:In-cell NMR[J]. BBA-Mol Basis Dis, 2017, 1865(5): 547-557. |
| [80] | PIELAK G J, TIAN F. Membrane proteins, magic-angle spinning, and in-cell NMR[J]. P Natl Acad Sci USA, 2012, 109(13): 4715-4716. DOI: 10.1073/pnas.1201502109. |
| [81] | FREEDBERG DI, SELENKO P. Live Cell NMR[J]. Annu Rev Biophy, 2014, 43: 171-192. DOI: 10.1146/annurev-biophys-051013-023136. |
| [82] | TOCHIO H. Watching protein structure at work in living cells using NMR spectroscopy[J]. Curr Opin Chem Biol, 2012, 16(5/6): 609-613. |
| [83] | LI C G, WANG G F, WANG Y Q, et al. Protein 19F NMR in Escherichia coli[J]. J Am Chem Soc, 2010, 132(1): 321. DOI: 10.1021/ja907966n. |
| [84] | BRINDLE K, WILLIAMS SP, BOULTON M. 19F NMR detection of a fluorine-labelled enzyme in vivo[J]. Febs Lett, 1989, 255(1): 121-124. DOI: 10.1016/0014-5793(89)81073-7. |
| [85] | TAKECHI-HARAYA Y, AKI K, TOHYAMA Y, et al. Glycosaminoglycan binding and non-endocytic membrane translocation of cell-permeable octaarginine monitored by real-time in-cell nmr spectroscopy[J]. Pharmaceuticals (Basel), 2017, 10(2): 42. |
| [86] | LI D, ZHANG Y N, HE Y, et al. Protein-protein interaction analysis in crude bacterial lysates using combinational method of 19F site-specific incorporation and 19F NMR[J]. Protein Cell, 2017, 8(2): 149-154. DOI: 10.1007/s13238-016-0336-8. |
| [87] | YODER N C, KUMAR K. Fluorinated amino acids in protein design and engineering[J]. Chem Soc Rev, 2002, 31(6): 335-341. DOI: 10.1039/b201097f. |
| [88] | JACKEL C, KOKSCH B. Fluorine in peptide design and protein engineering[J]. Eur J Org Chem, 2010, 2010(21): 4483-4503. |
| [89] | QIU X L, QING F L. Recent advances in the synthesis of fluorinated amino acids[J]. Eur J Org Chem, 2011, 2011(18): 3261-3278. DOI: 10.1002/ejoc.201100032. |
| [90] | SALWICZEK M, NYAKATURA EK, GERLING UIM, et al. Fluorinated amino acids:compatibility with native protein structures and effects on protein-protein interactions[J]. Chem Soc Rev, 2012, 41(6): 2135-2171. DOI: 10.1039/C1CS15241F. |
| [91] | ODAR C, WINKLER M, WILTSCHI B. Fluoro amino acids:A rarity in nature, yet a prospect for protein engineering[J]. Biotechnol J, 2015, 10(3): 427-446. DOI: 10.1002/biot.v10.3. |
| [92] | LIU Y X, ROSE J, HUANG S J, et al. A pH-gated conformational switch regulates the phosphatase activity of bifunctional HisKa-family histidine kinases[J]. Nat Commun, 2017, 8: 2104. DOI: 10.1038/s41467-017-02310-9. |
| [93] | KIRSCH P. Modern fluoroorganic chemistry:synthesis, reactivity, applications[M]. Wiley-VCH, 2006. |
| [94] | XIAO G Y, PARSONS J F, TESH K, et al. Conformational changes in the crystal structure of rat glutathione transferase M1-1 with global substitution of 3-fluorotyrosine for tyrosine[J]. J Mol Biol, 1998, 281(2): 323-339. DOI: 10.1006/jmbi.1998.1935. |
 2019, Vol. 36
2019, Vol. 36 
