2. University of Chinese Academy of Sciences, Beijing 100049, China
2. 中国科学院大学, 北京 100049
Surfactants are a kind of amphiphilic organic compounds, which have the ability of forming ordered aggregates and reducing the surface tension in aqueous solutions[1-4]. In most practical applications, mixtures of surfactants, rather than individual surfactants, are used in industries and life, because surfactant mixtures with proper additives can make the critical micelle concentration (CMC) values decrease and improve the surface activity, which have better performances and called "synergistic effect"[5-7]. The regular solution theory developed by Rubingh[8] has been widely used in most mixed binary surfactant systems[9-15]. But this theory lacks accuracy for its dependence on "the CMC of the mixed surfactant solution", which is not appropriate, because each surfactant of the mixtures has its own individual behavior. Multi-component systems which consist of nonionic, cationic and anionic surfactants are widely used as detergents, emulsifiers, chemical flooding agents, etc. Triton X-100 (TX-100), quaternary ammonium salt surfactants and sulfate surfactants are typical of these three categories of surfactants, because they are accessible with simple structures and good performances[16-24].
In comparison with the other experimental techniques available for studying the surfactant mixtures, nuclear magnetic resonance (NMR) spectroscopy has some advantages of not only providing microscopic information at molecular and atomic scale but also giving a chance to observe separately behaviors of each individual surfactant in the mixture at the same time[25-30].
In this work, an NMR approach is utilized to study the binary ionic/nonionic surfactant mixtures that consist of non-ionic surfactants, quaternary ammonium salt surfactants and sulfate surfactants, to evaluate the essence of the interaction in mixing the surfactants and provide a reference for choosing proper kinds and proportions of surfactants to be mixed, to get giving the best performance needed.
1 Experimental section 1.1 NMR experimentsAll NMR experiments were performed at 298.0 K on a Bruker AVANCE spectrometer with proton resonance frequency of 500.13 MHz. A small pulse flip-angle 30˚ was used rather than 90˚ in the conventional single pulse sequence, to reduce the time and get better signal-to-noise ratio. The NMR resonance peaks were ascertained through the peak of water (δ 4.70) as reference.
1.2 Reagents and materialsD2O (with a deuteration of 99.9%) was the product of Sigma-Aldrich, surfactants cetyltrimethylammonium bromide [CTAB, molecular weight (MW)=364.45], tetradecyltrimethylammonium bromide (TTAB, MW=336.39), dodecayltrimethylammonium bromide (DTAB, MW=308.34), TX-100 (MW=646.86), sodium hexadecyl sulfate (SHS, MW=344.49), and sodium dodecyl sulfate (SDS, MW=288.38) and polyethylene glycol (23) lauryl ether (Brij-35, MW=1 199.54) were the products of Acros Organics, TCI, Sinopharm Chemical Reagent, Nacalai Tesque, Adamas-beta, Alfa Aesar and J. T. Baker, respectively. The above mentioned reagents were used without further purification as received. Chemical structures and proton numbering of TX-100, Brij-35, CTAB, TTAB, DTAB, SHS, and SDS are shown in Fig. 1. The high concentration solutions of single surfactants were prepared by D2O and mixed in some certain proportions, then the mixed solutions are diluted by D2O to get different concentrations of binary systems.
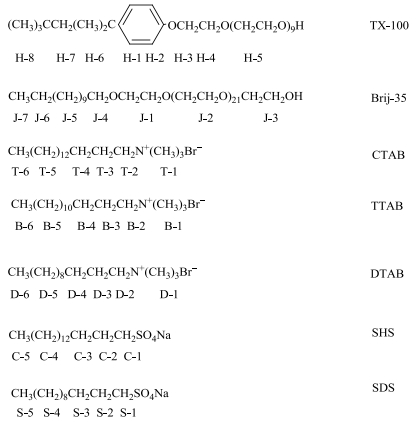
|
Fig. 1 Chemical structures and proton numbering of TX-100, Brij-35, CTAB, TTAB, DTAB, SHS and SDS |
According to the variation of chemical shift (δ) of characteristic peaks H-1, J-4, T-6, B-6, D-6, C-1 and S-1 against the reciprocal of concentrations, CMC values for TX-100, Brij-35, CTAB, TTAB, DTAB, SHS, and SDS in various mixed solutions were calculated, respectively (The calculation method was shown in Supporting Information that is available on the CJMR website at doi: 10.11938/cjmr20172610). The results of which have been depicted in Fig. 2 and 3.

|
Fig. 2 CMC values of CTAB and TX-100 in CTAB/TX-100 mixtures (a), CMC values of TTAB and TX-100 in TTAB/TX-100 mixtures (b), CMC values of DTAB and TX-100 in DTAB/TX-100 mixtures (c), CMC values of SHS and TX-100 in SHS/TX-100 mixtures (d), CMC values of SDS and TX-100 in SDS/TX-100 mixtures (e), CMC values of CTAB and Brij-35 in CTAB/Brij-35 mixtures (f) against the mole fractions of ionic surfactant in binary systems |
|
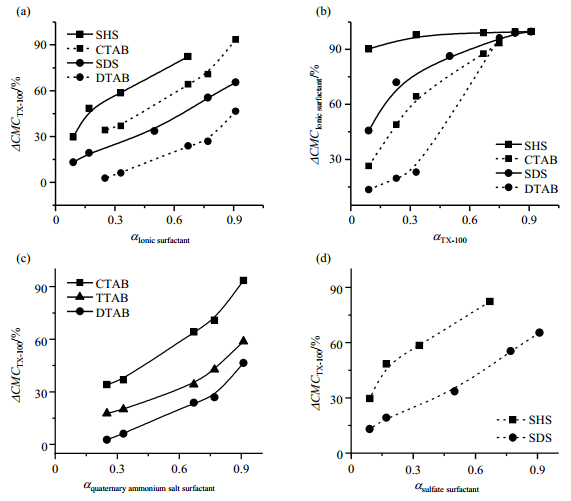
Fig. 3 The percentage changes of CMC values of TX-100 that mixed with different ionic surfactants (a), of different ionic surfactants that mixed with TX-100 (b), of TX-100 that mixed with different quaternary ammonium salt surfactants (c), of TX-100 that mixed with different sulfate surfactants (d) |
From Fig. 2(a)~2(f), it can be found that the CMC values of ionic CTAB, TTAB, DTAB, SHS or SDS decrease with the decrease in their mole factions (with the increase in the mole factions of nonionic TX-100 or Brij-35). At the same time, CMC values of TX-100 or Brij-35 decrease with the increase in the mole factions of ionic surfactants. It suggests that mixing two surfactants results in a decrease in the CMC values of the mixed surfactants by their partners, respectively. As well known, a part of surfactant molecules in aqueous solutions are adsorbed at the interface between the aqueous and the air phase, the adsorbed molecules exchange fast with the molecules staying in the solution. They are in dynamic equilibrium with a definite equilibrium adsorption constant[5, 31]. If a second surfactant is added, it would also be adsorbed at the interface, thus will replace a part of the already adsorbed molecules of the first surfactant, resulting in a decrease in the first surfactant molecules per unit area of the adsorption area. In order to reestablish the adsorption equilibrium, the concentration of the first surfactant in the solution should be decreased, so the rest molecules of the first surfactant in the bulk solution are caused to aggregate forming micelles. This is the reason why the CMC values of mixed surfactants decreased. This fact clearly reveals the essence of "synergistic effect" simply.
Through carefully examining, one finds that the extents of CMC values decreased by their partners are different in different surfactant pairs. Mixing with anionic surfactant, SDS and SHS, the decrease in CMC of TX-100 is more pronounced than when mixing with the cationic ones which have the same hydrophobic chains, respectively [Fig. 3(a) and 3(b)]. It can be understood that the electron cloud of the orbital of the phenyl group of TX-100 attracts the positively charged head group of surfactants, resulting in a decrease in space between cationic surfactants and TX-100 molecules. In other words, the area occupied by this couple of molecules, becomes smaller than the sum of the area of their individual molecules. On the contrary, the π orbital electron cloud of the phenyl group of TX-100 repels the negatively charged head group of surfactants, resulting in an increase in space between anionic surfactants and TX-100 molecules. The area occupied by this couples of molecules, becomes larger than the sum of the area of their individual molecules. Consequently, SDS or SHS replaces more TX-100 molecules adsorbed on the interface, it shows more pronounced effect in decreasing the CMC of TX-100. The more TX-100 adsorbed molecules are repelled from the interface adsorbed monolayer by mixing with anionic surfactants, the more obvious decrease in CMC of TX-100.
It should be mentioned that the electro-interaction is not the only factor influencing the degree of the CMC values decreased in binary surfactants. The length of the hydrophobic chains also shows some influence [Fig. 3(c) and 3(d)]. The longer the hydrophobic chains of the ionic surfactants are, the more obvious the CMC values of the other surfactant decreased, which suggests a steric effect between the adsorbed molecules.
3 ConclusionBecause the macroscopic measurement methods (tensiometry, spectrophotomentry, conductometry, etc.) are not able to provide individual behavior of each component in the surfactant mixture, models with complicated function fitting have to be built to analyze the observed data. On the contrary, the use of NMR provides microscopic information about the behavior of individual surfactant in the mixture. The results show that mixing surfactants induce the decrease in CMC of each surfactant by their partners in the binary mixtures. Through the surface adsorption theory of surfactant in combination with concerning the electro-characteristics and the hydrophobic chain length of the mixed binary surfactants, the essence of the traditional term, often called "synergistic effect", is successfully clarified.
| [1] | PORTER M R. Handbook of Surfactants[M]. US: Springer, 2013. |
| [2] | LEE Y S. Molecular self-assembly in solution I: Micelles[M]. John Wiley & Sons, 2007. |
| [3] | MYERS D. Surfactant science and technology[M]. John Wiley & Sons, 2005. |
| [4] | MOROI Y. Micelles: theoretical and applied aspects[M]. Springer Science & Business Media, 1992. |
| [5] | ROSEN M J, KUNJAPPU J T. Surfactants and interfacial phenomena[M]. John Wiley & Sons, 2012. |
| [6] | PROKHOROVA G V, GLUKHAREVA N A. Micellization in aqueous solutions of mixed surfactants containing alkylpolyglucosides[J]. Colloid J, 2011, 73(6): 841-845. |
| [7] | SHILOACH A, BLANKSCHTEIN D. Predicting micellar solution properties of binary surfactant mixtures[J]. Langmuir, 1998, 14(7): 1618-1636. |
| [8] | RUBINGH D N. Solution chemistry of surfactants[M]. New York: Springer, 1979. |
| [9] | PATEL U, PAREKH P, SASTRY N V, et al. Surface activity, micellization and solubilization of cationic gemini surfactant-conventional surfactants mixed systems[J]. J Mol Liq, 2017, 225: 888-896. |
| [10] | AZUM N, RUB M A, ASIRI A M. Experimental and theoretical approach to mixed surfactant system of cationic gemini surfactant with nonionic surfactant in aqueous medium[J]. J Mol Liq, 2014, 196: 14-20. |
| [11] | SZYMCZYK K, JANCZUK B. The properties of a binary mixture of nonionic surfactants in water at the water/air interface[J]. Langmuir, 2007, 23(9): 4972-4981. |
| [12] | ZHOU Q, ROSEN M J. Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: The regular solution approach[J]. Langmuir, 2003, 19(11): 4555-4562. |
| [13] | HOFFMANN H, POSSNECKER G. The mixing behavior of surfactants[J]. Langmuir, 1994, 10(2): 381-389. |
| [14] | ROSEN M J, HUA X Y. Synergism in binary mixtures of surfactants: 2. Some experimental data[J]. J Am Oil Chem Soc, 1982, 59(12): 582-585. DOI: 10.1007/BF02636329. |
| [15] | HUA X Y, ROSEN M J. Synergism in binary mixtures of surfactants: 1. Theoretical analysis[J]. J Colloid Interf Sci, 1982, 90(1): 212-219. DOI: 10.1016/0021-9797(82)90414-3. |
| [16] | THAKKAR K, BHARATIYA B, RAY D, et al. Molecular interactions involving aqueous Triton X-100 micelles and anionic surfactants: Investigations on surface activity and morphological transitions[J]. J Mol Liq, 2016, 223: 611-620. |
| [17] | PAREKH P, VARADE D, PARIKH J, et al. Anionic-cationic mixed surfactant systems: Micellar interaction of sodium dodecyl trioxyethylene sulfate with cationic gemini surfactants[J]. Colloids Surfaces A, 2011, 385(1/2/3): 111-120. |
| [18] | KUME G, GALLOTTI M, NUNES G. Review on anionic/cationic surfactant mixtures[J]. J Surfactants Deterg, 2008, 11(1): 1-11. |
| [19] | DAS C, CHAKRABORTY T, GHOSH S, et al. Mixed micellization of anionic-nonionic surfactants in aqueous media: a physicochemical study with theoretical consideration[J]. Colloid Polym Sci, 2008, 286(10): 1143-1155. |
| [20] | DAR A A, RATHER G M, DAS A R. Mixed micelle formation and solubilization behavior toward polycyclic aromatic hydrocarbons of binary and ternary cationic-nonionic surfactant mixtures[J]. J Phys Chem B, 2007, 111(12): 3122-3132. |
| [21] | LU T, HAN F, LI Z C, et al. Transitions of organized assemblies in mixed systems of cationic bolaamphiphile and anionic conventional surfactants[J]. Langmuir, 2006, 22(5): 2045-2049. |
| [22] | CHAKRABORTY H, SARKAR M. Optical spectroscopic and TEM studies of catanionic micelles of CTAB/SDS and their interaction with a NSAID[J]. Langmuir, 2004, 20(9): 3551-3558. |
| [23] | YAN Y, HUANG J B, LI Z C, et al. Aggregates transition depending on the concentration in the cationic bolaamphiphile/SDS mixed systems[J]. Langmuir, 2003, 19(3): 972-974. |
| [24] | SHIOI A, HATTON T A. Model for formation and growth of vesicles in mixed anionic/cationic (SOS/CTAB) surfactant systems[J]. Langmuir, 2002, 18(20): 7341-7348. |
| [25] | CUI X H, JIANG Y, YANG C S, et al. Mechanism of the mixed surfactant micelle formation[J]. J Phys Chem B, 2010, 114(23): 7808-7816. |
| [26] | YANG Q Q, ZHOU Q, SOMASUNDARAN P. NMR study of micellar microstructures of cationic single-chain and gemini surfactants and their mixtures with nonionic surfactant n-dodecyl-beta-D-maltoside[J]. Colloids Surfaces A, 2008, 322(1/2/3): 40-46. |
| [27] | CUI X H, MAO S Z, LIU M L, et al. Mechanism of surfactant micelle formation[J]. Langmuir, 2008, 24(19): 10771-10775. |
| [28] | GHARIBI H, JAVADIAN S, SOHRABI B, et al. Investigation of interaction parameters in mixed micelle using pulsed field gradient NMR spectroscopy[J]. J Colloid Inter Sci, 2005, 285(1): 351-359. |
| [29] |
YU G J, LIU J, MAO S Z, et al. Exchange kinetics of surfactants TX-100 and CTAB investigated by 1H NMR spectroscopy[J].
Chinese J Magn Reson, 2016, 33(3): 422-431.
俞刚金, 刘君, 毛诗珍, 等. TX-100和CTAB交换动力学的核磁共振研究[J]. 波谱学杂志, 2016, 33(3): 422-431. |
| [30] |
YANG C S, CUI X H, JIANG Y, et al. Mixed micelles of sodium dodecyl sulfate and triton X-100 in aqueous solution studied by 1H NMR[J].
Chinese J Magn Reson, 2009, 26(4): 466-475.
杨春升, 崔晓红, 蒋艳, 等. 表面活性剂SDS/TX-100混合体系的NMR研究[J]. 波谱学杂志, 2009, 26(4): 466-475. |
| [31] | KARAKASHEV S I, NGUYEN A V, MILLER J D. Equilibrium adsorption of surfactants at the gas-liquid interface[M]. Berlin Heidelberg: Springer, 2008: 25-55. |
 2019, Vol. 36
2019, Vol. 36 
